A non-human primate model for gluten sensitivity
- PMID: 18286171
- PMCID: PMC2229647
- DOI: 10.1371/journal.pone.0001614
A non-human primate model for gluten sensitivity
Abstract
Background and aims: Gluten sensitivity is widespread among humans. For example, in celiac disease patients, an inflammatory response to dietary gluten leads to enteropathy, malabsorption, circulating antibodies against gluten and transglutaminase 2, and clinical symptoms such as diarrhea. There is a growing need in fundamental and translational research for animal models that exhibit aspects of human gluten sensitivity.
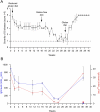
Methods: Using ELISA-based antibody assays, we screened a population of captive rhesus macaques with chronic diarrhea of non-infectious origin to estimate the incidence of gluten sensitivity. A selected animal with elevated anti-gliadin antibodies and a matched control were extensively studied through alternating periods of gluten-free diet and gluten challenge. Blinded clinical and histological evaluations were conducted to seek evidence for gluten sensitivity.
Results: When fed with a gluten-containing diet, gluten-sensitive macaques showed signs and symptoms of celiac disease including chronic diarrhea, malabsorptive steatorrhea, intestinal lesions and anti-gliadin antibodies. A gluten-free diet reversed these clinical, histological and serological features, while reintroduction of dietary gluten caused rapid relapse.
Conclusions: Gluten-sensitive rhesus macaques may be an attractive resource for investigating both the pathogenesis and the treatment of celiac disease.
Conflict of interest statement
Figures


References
-
- Dicke WK, Weijers HA, Van De Kamer JH. Coeliac disease. II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr. 1953;42:34–42. - PubMed
-
- Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–298. - PubMed
-
- Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207–221. - PubMed
-
- Spurkland A, Ingvarsson G, Falk ES, Knutsen I, Sollid LM, et al. Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA-DQ (alpha 1*0501, beta 1*02) or the HLA-DQ (alpha 1*03, beta 1*0302) heterodimers. Tissue Antigens. 1997;49:29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

