Human VRK1 is an early response gene and its loss causes a block in cell cycle progression
- PMID: 18286197
- PMCID: PMC2241669
- DOI: 10.1371/journal.pone.0001642
Human VRK1 is an early response gene and its loss causes a block in cell cycle progression
Abstract
Background: In mammalian cells regulatory proteins controlling the cell cycle are necessary due to the requirements of living in a heterogeneous environment of cell-interactions and growth factors. VRK1 is a novel serine-threonine kinase that phosphorylates several transcription factors and is associated with proliferation phenotypes.
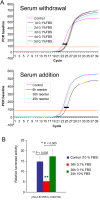
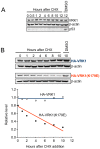
Methodology/principal findings: In this report VRK1 has been identified as regulated in the cell cycle. VRK1 gene expression is activated by the addition of serum to starved cells, indicating it is required for the exit of G0 phase and entry in G1; a response that parallels the re-expression of MYC, FOS and CCND1 (cyclin D1) genes, suggesting that VRK1 is an early-response gene. VRK1 gene expression is also shutdown by serum withdrawal. The human VRK1 gene promoter cloned in a luciferase reporter responds similarly to serum. In response to serum, the level of VRK1 protein expression has a positive correlation with cell proliferation markers such as phosphorylated-Rb or PCNA, and is inversely correlated with cell cycle inhibitors such as p27. The elimination of VRK1 by siRNA results in a G1 block in cell division, and in loss of phosphorylated-Rb, cyclin D1, and other proliferation markers. Elimination of VRK1 by siRNA induces a reduction of cell proliferation. VRK1 colocalizes with p63 in proliferating areas of squamous epithelium, and identifies a subpopulation in the basal layer.
Conclusions/significance: VRK1 is an immediate early response gene required for entry in G1, and due to its implication in normal cell proliferation and division, might be a new target for development of inhibitors of cellular proliferation.
Conflict of interest statement
Figures






Similar articles
-
Oncogenic Sox2 regulates and cooperates with VRK1 in cell cycle progression and differentiation.Sci Rep. 2016 Jun 23;6:28532. doi: 10.1038/srep28532. Sci Rep. 2016. PMID: 27334688 Free PMC article.
-
Vaccinia-related kinase 1 promotes hepatocellular carcinoma by controlling the levels of cell cycle regulators associated with G1/S transition.Oncotarget. 2015 Oct 6;6(30):30130-48. doi: 10.18632/oncotarget.4967. Oncotarget. 2015. PMID: 26375549 Free PMC article.
-
VRK1 phosphorylates CREB and mediates CCND1 expression.J Cell Sci. 2008 Sep 15;121(Pt 18):3035-41. doi: 10.1242/jcs.026757. Epub 2008 Aug 19. J Cell Sci. 2008. PMID: 18713830
-
Nuclear functions regulated by the VRK1 kinase.Nucleus. 2024 Dec;15(1):2353249. doi: 10.1080/19491034.2024.2353249. Epub 2024 May 16. Nucleus. 2024. PMID: 38753965 Free PMC article. Review.
-
The human VRK1 chromatin kinase in cancer biology.Cancer Lett. 2021 Apr 10;503:117-128. doi: 10.1016/j.canlet.2020.12.032. Epub 2021 Jan 29. Cancer Lett. 2021. PMID: 33516791 Review.
Cited by
-
Hypoxia and miscoupling between reduced energy efficiency and signaling to cell proliferation drive cancer to grow increasingly faster.J Mol Cell Biol. 2012 Jun;4(3):174-6. doi: 10.1093/jmcb/mjs017. Epub 2012 Apr 20. J Mol Cell Biol. 2012. PMID: 22523396 Free PMC article. No abstract available.
-
VRK1 regulates Cajal body dynamics and protects coilin from proteasomal degradation in cell cycle.Sci Rep. 2015 Jun 12;5:10543. doi: 10.1038/srep10543. Sci Rep. 2015. PMID: 26068304 Free PMC article.
-
VRK2 inhibits mitogen-activated protein kinase signaling and inversely correlates with ErbB2 in human breast cancer.Mol Cell Biol. 2010 Oct;30(19):4687-97. doi: 10.1128/MCB.01581-09. Epub 2010 Aug 2. Mol Cell Biol. 2010. PMID: 20679487 Free PMC article.
-
Overexpression of the VRK1 kinase, which is associated with breast cancer, induces a mesenchymal to epithelial transition in mammary epithelial cells.PLoS One. 2018 Sep 4;13(9):e0203397. doi: 10.1371/journal.pone.0203397. eCollection 2018. PLoS One. 2018. PMID: 30180179 Free PMC article.
-
Analysis of kinase gene expression patterns across 5681 human tissue samples reveals functional genomic taxonomy of the kinome.PLoS One. 2010 Dec 3;5(12):e15068. doi: 10.1371/journal.pone.0015068. PLoS One. 2010. PMID: 21151926 Free PMC article.
References
-
- Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol. 1991;2:213–222. - PubMed
-
- Olashaw N, Pledger WJ. Paradigms of growth control: relation to Cdk activation. Sci STKE. 2002;2002:RE7. - PubMed
-
- Malumbres M, Hunt SL, Sotillo R, Martin J, Odajima J, et al. Driving the cell cycle to cancer. Adv Exp Med Biol. 2003;532:1–11. - PubMed
-
- Sanchez I, Dynlacht BD. New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol. 2005;16:311–321. - PubMed
-
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

