Precancerous stem cells can serve as tumor vasculogenic progenitors
- PMID: 18286204
- PMCID: PMC2242848
- DOI: 10.1371/journal.pone.0001652
Precancerous stem cells can serve as tumor vasculogenic progenitors
Abstract
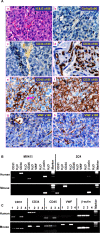
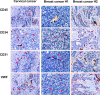
Tumor neo-vascularization is critical for tumor growth, invasion and metastasis, which has been considered to be mediated by a mechanism of angiogenesis. However, histopathological studies have suggested that tumor cells might be the progenitor for tumor vasculature. Recently, we have reported that the precancerous stem cells (pCSCs) representing the early stage of developing cancer stem cells (CSCs), have the potential for both benign and malignant differentiation. Therefore, we investigated whether pCSCs serve as progenitors for tumor vasculogenesis. Herein, we report that in the pCSC-derived tumors, most blood vessels were derived from pCSCs. Some pCSCs constitutively expressed vasculogenic receptor VEGFR-2, which can be up-regulated by hypoxia and angiogenesis-promoting cytokines, such as GM-CSF, Flt3 ligand, and IL-13. The pCSCs are much more potent in tumor vasculogenesis than the differentiated tumor monocytic cells (TMCs) from the same tumor, which had comparable or even higher capacity to produce some vascular growth factors, suggesting that the potent tumor vasculogenesis of pCSCs is associated with their intrinsic stem-like property. Consistently tumor vasculogenesis was also observed in human cancers such as cervical cancer and breast cancer and xenograft lymphoma. Our studies indicate that pCSCs can serve as tumor vasculogenic stem/progenitor cells (TVPCs), and may explain why anti-angiogenic cancer therapy trials are facing challenge.
Conflict of interest statement
Figures





References
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. - PubMed
-
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

