Optimization of volumetric computed tomography for skeletal analysis of model genetic organisms
- PMID: 18286615
- PMCID: PMC3189451
- DOI: 10.1002/ar.20670
Optimization of volumetric computed tomography for skeletal analysis of model genetic organisms
Abstract
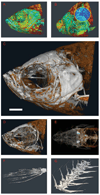
Forward and reverse genetics now allow researchers to understand embryonic and postnatal gene function in a broad range of species. Although some genetic mutations cause obvious morphological change, other mutations can be more subtle and, without adequate observation and quantification, might be overlooked. For the increasing number of genetic model organisms examined by the growing field of phenomics, standardized but sensitive methods for quantitative analysis need to be incorporated into routine practice to effectively acquire and analyze ever-increasing quantities of phenotypic data. In this study, we present platform-independent parameters for the use of microscopic x-ray computed tomography (microCT) for phenotyping species-specific skeletal morphology of a variety of different genetic model organisms. We show that microCT is suitable for phenotypic characterization for prenatal and postnatal specimens across multiple species.
Figures





References
-
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. - PubMed
-
- Attwood D. Microscopy: nanotomography comes of age. Nature. 2006;442:642–643. - PubMed
-
- Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N, Hicks G, Insel TR, Joyner A, Koller BH, Lloyd KC, Magnuson T, Moore MW, Nagy A, Pollock JD, Roses AD, Sands AT, Seed B, Skarnes WC, Snoddy J, Soriano P, Stewart DJ, Stewart F, Stillman B, Varmus H, Varticovski L, Verma IM, Vogt TF, von Melchner H, Witkowski J, Woychik RP, Wurst W, Yancopoulos GD, Young SG, Zambrowicz B. The knockout mouse project. Nat Genet. 2004;36:921–924. - PMC - PubMed
-
- Beltran WA, Vanore M, Ollivet F, Nemoz-Bertholet F, Aujard F, Clerc B, Chahory S. Ocular findings in two colonies of gray mouse lemurs (Microcebus murinus) Vet Ophthalmol. 2007;10:43–49. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

