The ABCA1 cholesterol transporter associates with one of two distinct dystrophin-based scaffolds in Schwann cells
- PMID: 18286648
- PMCID: PMC4335170
- DOI: 10.1002/glia.20636
The ABCA1 cholesterol transporter associates with one of two distinct dystrophin-based scaffolds in Schwann cells
Abstract
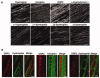
Cytoskeletal scaffolding complexes help organize specialized membrane domains with unique functions on the surface of cells. In this study, we define the scaffolding potential of the Schwann cell dystrophin glycoprotein complex (DGC) by establishing the presence of four syntrophin isoforms, (alpha1, beta1, beta2, and gamma2), and one dystrobrevin isoform, (alpha-dystrobrevin-1), in the abaxonal membrane. Furthermore, we demonstrate the existence of two separate DGCs in Schwann cells that divide the abaxonal membrane into spatially distinct domains, the DRP2/periaxin rich plaques and the Cajal bands that contain Dp116, utrophin, alpha-dystrobrevin-1 and four syntrophin isoforms. Finally, we show that the two different DGCs can scaffold unique accessory molecules in distinct areas of the Schwann cell membrane. Specifically, the cholesterol transporter ABCA1, associates with the Dp116/syntrophin complex in Cajal bands and is excluded from the DRP2/periaxin rich plaques.
(c) 2008 Wiley-Liss, Inc.
Figures


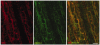


References
-
- Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993;11(3):531–40. - PubMed
-
- Albrecht DE, Froehner SC. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals. 2002;11(3):123–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

