Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity
- PMID: 18287029
- PMCID: PMC2268581
- DOI: 10.1073/pnas.0711741105
Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity
Abstract
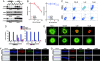
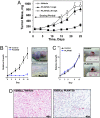
BRAF(V600E) is the most frequent oncogenic protein kinase mutation known. Furthermore, inhibitors targeting "active" protein kinases have demonstrated significant utility in the therapeutic repertoire against cancer. Therefore, we pursued the development of specific kinase inhibitors targeting B-Raf, and the V600E allele in particular. By using a structure-guided discovery approach, a potent and selective inhibitor of active B-Raf has been discovered. PLX4720, a 7-azaindole derivative that inhibits B-Raf(V600E) with an IC(50) of 13 nM, defines a class of kinase inhibitor with marked selectivity in both biochemical and cellular assays. PLX4720 preferentially inhibits the active B-Raf(V600E) kinase compared with a broad spectrum of other kinases, and potent cytotoxic effects are also exclusive to cells bearing the V600E allele. Consistent with the high degree of selectivity, ERK phosphorylation is potently inhibited by PLX4720 in B-Raf(V600E)-bearing tumor cell lines but not in cells lacking oncogenic B-Raf. In melanoma models, PLX4720 induces cell cycle arrest and apoptosis exclusively in B-Raf(V600E)-positive cells. In B-Raf(V600E)-dependent tumor xenograft models, orally dosed PLX4720 causes significant tumor growth delays, including tumor regressions, without evidence of toxicity. The work described here represents the entire discovery process, from initial identification through structural and biological studies in animal models to a promising therapeutic for testing in cancer patients bearing B-Raf(V600E)-driven tumors.
Conflict of interest statement
Conflict of interest statement: S.-H.K. and J.S. are Plexxikon founders and share holders.
Figures
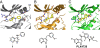
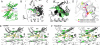
References
-
- Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Samowitz WS, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. - PubMed
-
- Riesco-Eizaguirre G, et al. The oncogene BRAFV600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. - PubMed
-
- Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

