Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides
- PMID: 18287037
- PMCID: PMC2268539
- DOI: 10.1073/pnas.0708254105
Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides
Abstract
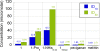
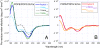
Antimicrobial peptides (AMPs) and their mimics are emerging as promising antibiotic agents. We present a library of "ampetoids" (antimicrobial peptoid oligomers) with helical structures and biomimetic sequences, several members of which have low-micromolar antimicrobial activities, similar to cationic AMPs like pexiganan. Broad-spectrum activity against six clinically relevant BSL2 pathogens is also shown. This comprehensive structure-activity relationship study, including circular dichroism spectroscopy, minimum inhibitory concentration assays, hemolysis and mammalian cell toxicity studies, and specular x-ray reflectivity measurements shows that the in vitro activities of ampetoids are strikingly similar to those of AMPs themselves, suggesting a strong mechanistic analogy. The ampetoids' antibacterial activity, coupled with their low cytotoxicity against mammalian cells, make them a promising class of antimicrobials for biomedical applications. Peptoids are biostable, with a protease-resistant N-substituted glycine backbone, and their sequences are highly tunable, because an extensive diversity of side chains can be incorporated via facile solid-phase synthesis. Our findings add to the growing evidence that nonnatural foldamers will emerge as an important class of therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

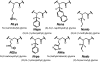



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

