Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: Implications for somatic embryogenesis
- PMID: 18287041
- PMCID: PMC2268600
- DOI: 10.1073/pnas.0712364105
Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: Implications for somatic embryogenesis
Abstract
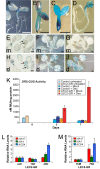
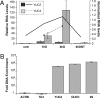
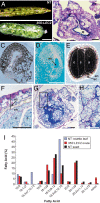
LEAFY COTYLEDON2 (LEC2) is a central regulator of embryogenesis sufficient to induce somatic cells to form embryos when expressed ectopically. Here, we analyze the cellular processes induced by LEC2, a B3 domain transcription factor, that may underlie its ability to promote somatic embryogenesis. We show auxin-responsive genes are induced after LEC2 activation in seedlings. Genes encoding enzymes involved in auxin biosynthesis, YUC2 and YUC4, are activated within 1 h after induction of LEC2 activity, and YUC4 appears to be a direct transcriptional target of LEC2. We also show ectopic LEC2 expression induces accumulation of seed storage protein and oil bodies in vegetative and reproductive organs, events that normally occur during the maturation phase of embryogenesis. Furthermore, LEC2 activates seed protein genes before an increase in RNAs encoding LEC1 or FUS3 is observed. Thus, LEC2 causes rapid changes in auxin responses and induces cellular differentiation characteristic of the maturation phase. The relevance of these changes to the ability of LEC2 to promote somatic embryogenesis is discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

