Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation
- PMID: 18287048
- PMCID: PMC2268587
- DOI: 10.1073/pnas.0708931105
Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation
Abstract
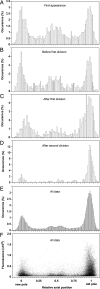
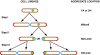
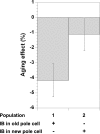
Aging, defined as a decrease in reproduction rate with age, is a fundamental characteristic of all living organisms down to bacteria. Yet we know little about the causal molecular mechanisms of aging within the in vivo context of a wild-type organism. One of the prominent markers of aging is protein aggregation, associated with cellular degeneracy in many age-related diseases, although its in vivo dynamics and effect are poorly understood. We followed the appearance and inheritance of spontaneous protein aggregation within lineages of Escherichia coli grown under nonstressed conditions using time-lapse microscopy and a fluorescently tagged chaperone (IbpA) involved in aggregate processing. The fluorescent marker is shown to faithfully identify in vivo the localization of aggregated proteins, revealing their accumulation upon cell division in cells with older poles. This accretion is associated with >30% of the loss of reproductive ability (aging) in these cells relative to the new-pole progeny, devoid of parental inclusion bodies, that exhibit rejuvenation. This suggests an asymmetric strategy whereby dividing cells segregate damage at the expense of aging individuals, resulting in the perpetuation of the population.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

