Generation of human induced pluripotent stem cells from dermal fibroblasts
- PMID: 18287077
- PMCID: PMC2268554
- DOI: 10.1073/pnas.0711983105
Generation of human induced pluripotent stem cells from dermal fibroblasts
Abstract
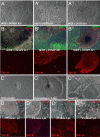
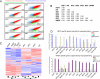
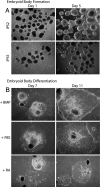
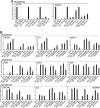
The generation of patient-specific pluripotent stem cells has the potential to accelerate the implementation of stem cells for clinical treatment of degenerative diseases. Technologies including somatic cell nuclear transfer and cell fusion might generate such cells but are hindered by issues that might prevent them from being used clinically. Here, we describe methods to use dermal fibroblasts easily obtained from an individual human to generate human induced pluripotent stem (iPS) cells by ectopic expression of the defined transcription factors KLF4, OCT4, SOX2, and C-MYC. The resultant cell lines are morphologically indistinguishable from human embryonic stem cells (HESC) generated from the inner cell mass of a human preimplantation embryo. Consistent with these observations, human iPS cells share a nearly identical gene-expression profile with two established HESC lines. Importantly, DNA fingerprinting indicates that the human iPS cells were derived from the donor material and are not a result of contamination. Karyotypic analyses demonstrate that reprogramming of human cells by defined factors does not induce, or require, chromosomal abnormalities. Finally, we provide evidence that human iPS cells can be induced to differentiate along lineages representative of the three embryonic germ layers indicating the pluripotency of these cells. Our findings are an important step toward manipulating somatic human cells to generate an unlimited supply of patient-specific pluripotent stem cells. In the future, the use of defined factors to change cell fate may be the key to routine nuclear reprogramming of human somatic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Byrne JA, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
-
- Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES cell-like state. Nature. 2007;448:318–324. - PubMed
-
- Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

