Transforming growth factor-beta1 (TGFbeta1) stimulates connective tissue growth factor (CCN2/CTGF) expression in human gingival fibroblasts through a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with forskolin block TGFbeta1-induced CCN2/CTGF expression
- PMID: 18287089
- PMCID: PMC2435243
- DOI: 10.1074/jbc.M710363200
Transforming growth factor-beta1 (TGFbeta1) stimulates connective tissue growth factor (CCN2/CTGF) expression in human gingival fibroblasts through a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with forskolin block TGFbeta1-induced CCN2/CTGF expression
Abstract
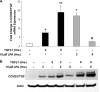
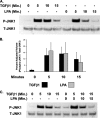
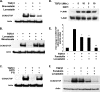
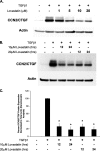
Regulation of connective tissue growth factor (CCN2/CTGF) in gingival fibroblasts is unique and may provide therapeutic opportunities to treat oral fibrotic diseases. RhoA was previously implicated in mediating the expression of CCN2/CTGF. We now present evidence that Rho family GTPases Rac1 and Cdc42 are the principal mediators of the transforming growth factor-beta1 (TGFbeta1)-stimulated expression of CCN2/CTGF in primary human gingival fibroblasts. TGFbeta1 does not stimulate RhoA activation in gingival fibroblasts, and the overexpression of dominant-negative RhoA does not reduce CCN2/CTGF expression in response to TGFbeta1. In contrast, the overexpression of dominant-negative forms of Cdc42 or Rac1 results in a dramatic reduction of CCN2/CTGF protein levels. Lovastatin and a geranylgeranyltransferase inhibitor reduce the TGFbeta1-stimulated levels of CCN2/CTGF protein by approximately 75 and 100%, respectively. We previously demonstrated that JNK1 phosphorylation by TGFbeta1 is also critical for TGFbeta1-induced CCN2/CTGF expression, and forskolin partially reduces levels of phosphorylated JNK1. Inhibition of geranylgeranyltransferase has no effect on levels of JNK phosphorylation in response to TGFbeta1 suggesting Rho-GTPases act independently of JNK1. The combination of lovastatin and forskolin results in a greater inhibitory effect than each agent alone and reduces CCN2/CTGF mRNA and protein expression by greater than 90%. This novel combination has additive inhibitory effects on the TGFbeta1-stimulated expression of CCN2/CTGF in human gingival fibroblasts through the simultaneous disruption of Rho- and JNK1-mediated pathways, respectively. This combination of available therapeutic compounds may therefore be useful in designing treatment strategies for oral fibrotic conditions in which gingival CCN2/CTGF is elevated.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

