Inhibition of CD1 antigen presentation by human cytomegalovirus
- PMID: 18287231
- PMCID: PMC2293051
- DOI: 10.1128/JVI.01447-07
Inhibition of CD1 antigen presentation by human cytomegalovirus
Abstract
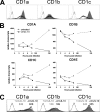
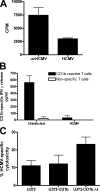
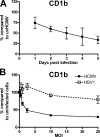
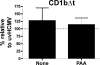
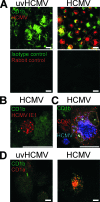
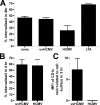
The betaherpesvirus human cytomegalovirus (HCMV) encodes several molecules that block antigen presentation by the major histocompatibility complex (MHC) proteins. Humans also possess one other family of antigen-presenting molecules, the CD1 family; however, the effect of HCMV on CD1 expression is unknown. The majority of CD1 molecules are classified on the basis of homology as group 1 CD1 and are present almost exclusively on professional antigen-presenting cells such as dendritic cells, which are a major target for HCMV infection and latency. We have determined that HCMV encodes multiple blocking strategies targeting group 1 CD1 molecules. CD1 transcription is strongly inhibited by the HCMV interleukin-10 homologue cmvIL-10. HCMV also blocks CD1 antigen presentation posttranscriptionally by the inhibition of CD1 localization to the cell surface. This function is not performed by a known HCMV MHC class I-blocking molecule and is substantially stronger than the blockage induced by herpes simplex virus type 1. Antigen presentation by CD1 is important for the development of the antiviral immune response and the generation of mature antigen-presenting cells. HCMV present in antigen-presenting cells thus blunts the immune response by the blockage of CD1 molecules.
Figures



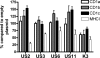

References
-
- Brigl, M., and M. B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22817-890. - PubMed
-
- Cao, X., M. Sugita, N. van der Wel, J. Lai, R. A. Rogers, P. J. Peters, and M. B. Brenner. 2002. CD1 molecules efficiently present antigen in immature dendritic cells and traffic independently of MHC class II during dendritic cell maturation. J. Immunol. 1694770-4777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

