Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs
- PMID: 18287245
- PMCID: PMC2293066
- DOI: 10.1128/JVI.02602-07
Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs
Abstract
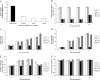
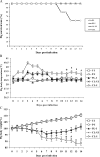
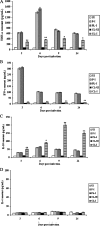
Alveolar macrophages constitutively reside in the respiratory tracts of pigs and humans. An in vivo role of alveolar macrophages in defending against influenza viruses in mice infected with a reassorted influenza virus, 1918 HA/NA:Tx/91, was reported, but there has been no report on an in vivo role of alveolar macrophages in a natural host such as a pig using currently circulating human influenza virus. Here we show that in vivo depletion of alveolar macrophages in pigs by dichloromethylene diphosphonate (MDPCL2) treatment results in 40% mortality when pigs are infected with currently circulating human H1N1 influenza viruses, while none of the infected control pigs died. All infected pigs depleted of alveolar macrophages suffered from more severe respiratory signs than infected control pigs. Induction of tumor necrosis factor alpha in the infected pigs depleted of alveolar macrophages was significantly lower than that in the lungs of infected control pigs, and the induction of interleukin-10, an immunosuppressive cytokine, significantly increased in the lungs of infected pigs depleted of alveolar macrophages compared to infected control pigs. When we measured antibody titers and CD8(+) T lymphocytes expressing gamma interferon (IFN-gamma), lower antibody titers and a lower percentage of CD8(+) T lymphocytes expressing IFN-gamma were detectable in MDPCL2-treated infected pigs than in phosphate-buffered saline- and liposome-treated and infected pigs. Taken together, our findings suggest that alveolar macrophages are essential for controlling H1N1 influenza viruses in pigs.
Figures



References
-
- Bancroft, J. D., and A. Stevens. 1996. Theory and practice of histological techniques, 4th ed. Churchill Livingstone, New York, NY.
-
- Bender, B. S., and P. A. Small, Jr. 1992. Influenza: pathogenesis and host defense. Semin. Respir. Infect. 738-45. - PubMed
-
- Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 3601831-1837. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

