Deletion of Epstein-Barr virus BFLF2 leads to impaired viral DNA packaging and primary egress as well as to the production of defective viral particles
- PMID: 18287246
- PMCID: PMC2293001
- DOI: 10.1128/JVI.02436-07
Deletion of Epstein-Barr virus BFLF2 leads to impaired viral DNA packaging and primary egress as well as to the production of defective viral particles
Abstract
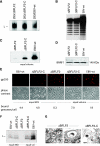
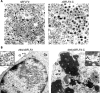
Previous genetic and biochemical studies performed with several members of the Alphaherpesvirus subfamily have shown that the UL31 and UL34 proteins are essential components of the molecular machinery that mediates the primary egress of newly assembled capsids across the nuclear membrane. Further, there is substantial evidence that BFLF2 and BFRF1, the respective positional homologs of UL31 and UL34 in the Epstein-Barr virus (EBV) genome, are also their functional homologs, i.e., that the UL31/UL34 pathway is common to distant herpesviruses. However, the low degree of protein sequence identity between UL31 and BFLF2 would argue against such a hypothesis. To further clarify this issue, we have constructed a recombinant EBV strain devoid of BFLF2 (DeltaBFLF2) and show that BFLF2 is crucial for efficient virus production but not for lytic DNA replication or B-cell transformation. This defective phenotype could be efficiently restored by trans complementation with a BFLF2 expression plasmid. Detailed analysis of replicating cells by electron microscopy revealed that, as expected, DeltaBFLF2 viruses not only failed to egress from the nucleus but also showed defective DNA packaging. Nonfunctional primary egress did not, however, impair the production and extracellular release of enveloped but empty viral particles that comprised L particles containing tegument-like structures and a few virus-like particles carrying empty capsids. The DeltaBFLF2 and DeltaUL31 phenotypes therefore only partly overlap, from which we infer that BFLF2 and UL31 have substantially diverged during evolution to fulfil related but distinct functions.
Figures

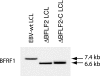
References
-
- Calderwood, M. A., K. Venkatesan, L. Xing, M. R. Chase, A. Vazquez, A. M. Holthaus, A. E. Ewence, N. Li, T. Hirozane-Kishikawa, D. E. Hill, M. Vidal, E. Kieff, and E. Johannsen. 2007. Epstein-Barr virus and virus human protein interaction maps. Proc. Natl. Acad. Sci. USA 1047606-7611. - PMC - PubMed
-
- Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1589-14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

