C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial
- PMID: 18287255
- PMCID: PMC2390949
- DOI: 10.2215/CJN.00480107
C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial
Abstract
Background and objectives: This study examined the efficacy of C.E.R.A., a continuous erythropoietin receptor activator, for correcting anemia in patients who had chronic kidney disease (CKD) and were not on dialysis.
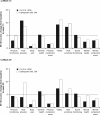
Design, setting, participants, & measurements: In this open-label, randomized, parallel-group, Phase III study, 324 adult patients with CKD not on dialysis nor receiving treatment with erythropoiesis-stimulating agents (ESAs) were randomly assigned (1:1) to receive subcutaneous C.E.R.A. once every 2 wk or darbepoetin alfa once weekly during an 18-wk correction period and a 10-wk evaluation period. Thereafter, patients receiving C.E.R.A. were randomly assigned to C.E.R.A. once every 2 wk or once monthly, and patients receiving darbepoetin alfa could receive darbepoetin alfa once weekly or once every 2 wk for a 24-wk extension period. Dosage was adjusted to achieve a hemoglobin (Hb) response and to maintain Hb +/-1 g/dl of the response level and 11 to 13 g/dl. Primary end points were Hb response rate during correction and evaluation and change in Hb concentration between baseline and evaluation.
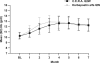
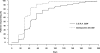
Results: Hb response rates were 97.5% for C.E.R.A. and 96.3% for darbepoetin alfa. Adjusted mean changes in Hb from baseline to evaluation were 2.15 g/dl (C.E.R.A.) and 2.00 g/dl (darbepoetin alfa). Analysis showed that C.E.R.A. once every 2 wk was as effective as darbepoetin alfa once weekly for correcting anemia. Hb levels remained stable in all groups during the extension period. C.E.R.A. and darbepoetin alfa were well tolerated.
Conclusions: Subcutaneous C.E.R.A. once every 2 wk corrects anemia in ESA-naïve patients who are not on dialysis.
Figures



References
-
- Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W: Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12: 2465–2473, 2001 - PubMed
-
- Levin A: Prevalence of cardiovascular damage in early renal disease. Nephrol Dial Transplant 16[Suppl 2]: 7–11, 2001 - PubMed
-
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 - PubMed
-
- Barrett BJ, Fenton SS, Ferguson B, Halligan P, Langlois S, Mccready WG, Muirhead N, Weir RV: Clinical practice guidelines for the management of anemia coexistent with chronic renal failure. Canadian Society of Nephrology. J Am Soc Nephrol 10[Suppl 13]: S292–S296, 1999 - PubMed
-
- Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougall IC, Macleod A, Wieçek A, Cameron S, European Best Practice Guidelines Working Group: Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19[Suppl 2]: ii1–ii47, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

