An input-representing interneuron regulates spike timing and thereby phase switching in a motor network
- PMID: 18287508
- PMCID: PMC6671438
- DOI: 10.1523/JNEUROSCI.4755-07.2008
An input-representing interneuron regulates spike timing and thereby phase switching in a motor network
Abstract
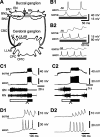
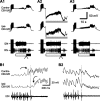
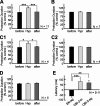
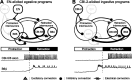
Despite the importance of spike-timing regulation in network functioning, little is known about this regulation at the cellular level. In the Aplysia feeding network, we show that interneuron B65 regulates the timing of the spike initiation of phase-switch neurons B64 and cerebral-buccal interneuron-5/6 (CBI-5/6), and thereby determines the identity of the neuron that acts as a protraction terminator. Previous work showed that B64 begins to fire before the end of protraction phase and terminates protraction in CBI-2-elicited ingestive, but not in CBI-2-elicited egestive programs, thus indicating that the spike timing and phase-switching function of B64 depend on the type of the central pattern generator (CPG)-elicited response rather than on the input used to activate the CPG. Here, we find that CBI-5/6 is a protraction terminator in egestive programs elicited by the esophageal nerve (EN), but not by CBI-2, thus indicating that, in contrast to B64, the spike timing and protraction-terminating function of CBI-5/6 depends on the input to the CPG rather than the response type. Interestingly, B65 activity also depends on the input in that B65 is highly active in EN-elicited programs, but not in CBI-2-elicited programs independent of whether the programs are ingestive or egestive. Notably, during EN-elicited egestive programs, hyperpolarization of B65 delays the onset of CBI-5/6 firing, whereas in CBI-2-elicited ingestive programs, B65 stimulation simultaneously advances CBI-5/6 firing and delays B64 firing, thereby substituting CBI-5/6 for B64 as the protraction terminator. Thus, we identified a neural mechanism that, in an input-dependent manner, regulates spike timing and thereby the functional role of specific neurons.
Figures







Similar articles
-
State dependence of spike timing and neuronal function in a motor pattern generating network.J Neurosci. 2007 Oct 3;27(40):10818-31. doi: 10.1523/JNEUROSCI.1806-07.2007. J Neurosci. 2007. PMID: 17913915 Free PMC article.
-
Neural mechanisms of motor program switching in Aplysia.J Neurosci. 2001 Sep 15;21(18):7349-62. doi: 10.1523/JNEUROSCI.21-18-07349.2001. J Neurosci. 2001. PMID: 11549745 Free PMC article.
-
B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica.J Neurophysiol. 1996 Apr;75(4):1327-44. doi: 10.1152/jn.1996.75.4.1327. J Neurophysiol. 1996. PMID: 8727381
-
Feeding neural networks in the mollusc Aplysia.Neurosignals. 2004 Jan-Apr;13(1-2):70-86. doi: 10.1159/000076159. Neurosignals. 2004. PMID: 15004426 Review.
-
Coping with variability in small neuronal networks.Integr Comp Biol. 2011 Dec;51(6):845-55. doi: 10.1093/icb/icr074. Epub 2011 Jun 30. Integr Comp Biol. 2011. PMID: 21724619 Free PMC article. Review.
Cited by
-
Convergent effects of neuropeptides on the feeding central pattern generator of Aplysia californica.J Neurophysiol. 2022 Jun 1;127(6):1445-1459. doi: 10.1152/jn.00025.2022. Epub 2022 May 4. J Neurophysiol. 2022. PMID: 35507477 Free PMC article.
-
Motor outputs in a multitasking network: relative contributions of inputs and experience-dependent network states.J Neurophysiol. 2009 Dec;102(6):3711-27. doi: 10.1152/jn.00844.2009. Epub 2009 Oct 21. J Neurophysiol. 2009. PMID: 19846618 Free PMC article.
-
Distinct inhibitory neurons exert temporally specific control over activity of a motoneuron receiving concurrent excitation and inhibition.J Neurosci. 2009 Sep 23;29(38):11732-44. doi: 10.1523/JNEUROSCI.3051-09.2009. J Neurosci. 2009. PMID: 19776260 Free PMC article.
-
Predicting adaptive behavior in the environment from central nervous system dynamics.PLoS One. 2008;3(11):e3678. doi: 10.1371/journal.pone.0003678. Epub 2008 Nov 7. PLoS One. 2008. PMID: 18989362 Free PMC article.
-
Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors.J Neurosci. 2010 Jan 6;30(1):131-47. doi: 10.1523/JNEUROSCI.3282-09.2010. J Neurosci. 2010. PMID: 20053896 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
