Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes
- PMID: 18287530
- PMCID: PMC2366872
- DOI: 10.1091/mbc.e07-09-0973
Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes
Abstract
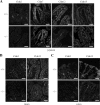
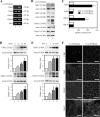
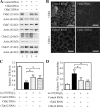
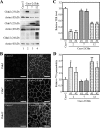
Ca(2+) is absorbed across intestinal epithelial monolayers via transcellular and paracellular pathways, and an active form of vitamin D(3), 1alpha,25-dihydroxyvitamin D(3) [1alpha,25(OH)(2)D(3)], is known to promote intestinal Ca(2+) absorption. However, the molecules driving the paracellular Ca(2+) absorption and its vitamin D dependency remain obscure. Because the tight junction proteins claudins are suggested to form paracellular channels for selective ions between neighboring cells, we hypothesized that specific intestinal claudins might facilitate paracellular Ca(2+) transport and that expression of these claudins could be induced by 1alpha,25(OH)(2)D(3). Herein, we show, by using RNA interference and overexpression strategies, that claudin-2 and claudin-12 contribute to Ca(2+) absorption in intestinal epithelial cells. We also provide evidence showing that expression of claudins-2 and -12 is up-regulated in enterocytes in vitro and in vivo by 1alpha,25(OH)(2)D(3) through the vitamin D receptor. These findings strongly suggest that claudin-2- and/or claudin-12-based tight junctions form paracellular Ca(2+) channels in intestinal epithelia, and they highlight a novel mechanism behind vitamin D-dependent calcium homeostasis.
Figures


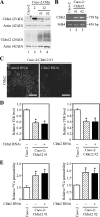
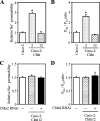
References
-
- Anderson J. M., Cereijido M. Introduction: evolution of ideas on the tight junction. In: Cereijido M., Anderson J. M., editors. Tight junctions. Boca Raton, FL: CRC Press; 2001. pp. 1–18.
-
- Alexandre M. D., Lu Q., Chen Y. H. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 2005;118:2683–2693. - PubMed
-
- Amasheh S., Meiri N., Gitter A. H., Schöneberg T., Mankertz J., Schulzke J. D., Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. - PubMed
-
- Bronner F., Pansu D., Stein W. D. An analysis of intestinal calcium transport across the rat intestine. Am. J. Physiol. Gastrointestinal. Liver Physiol. 1986;250:G561–G569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

