Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome
- PMID: 18287531
- PMCID: PMC2366880
- DOI: 10.1091/mbc.e07-09-0902
Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome
Abstract
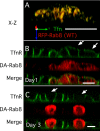
Rab8 is a monomeric GTPase that regulates the delivery of newly synthesized proteins to the basolateral surface in polarized epithelial cells. Recent publications have demonstrated that basolateral proteins interacting with the mu1-B clathrin adapter subunit pass through the recycling endosome (RE) en route from the TGN to the plasma membrane. Because Rab8 interacts with these basolateral proteins, these findings raise the question of whether Rab8 acts before, at, or after the RE. We find that Rab8 overexpression during the formation of polarity in MDCK cells, disrupts polarization of the cell, explaining how Rab8 mutants can disrupt basolateral endocytic and secretory traffic. However, once cells are polarized, Rab8 mutants cause mis-sorting of newly synthesized basolateral proteins such as VSV-G to the apical surface, but do not cause mis-sorting of membrane proteins already at the cell surface or in the endocytic recycling pathway. Enzymatic ablation of the RE also prevents traffic from the TGN from reaching the RE and similarly results in mis-sorting of newly synthesized VSV-G. We conclude that Rab8 regulates biosynthetic traffic through REs to the plasma membrane, but not trafficking of endocytic cargo through the RE. The data are consistent with a model in which Rab8 functions in regulating the delivery of TGN-derived cargo to REs.
Figures




Similar articles
-
The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells.J Cell Biol. 2003 Oct 27;163(2):339-50. doi: 10.1083/jcb.200307046. J Cell Biol. 2003. PMID: 14581456 Free PMC article.
-
Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane.J Cell Biol. 1993 Oct;123(1):35-45. doi: 10.1083/jcb.123.1.35. J Cell Biol. 1993. PMID: 8408203 Free PMC article.
-
Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins.EMBO J. 2007 Aug 22;26(16):3737-48. doi: 10.1038/sj.emboj.7601813. Epub 2007 Aug 2. EMBO J. 2007. PMID: 17673908 Free PMC article.
-
Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity.Histol Histopathol. 2009 Sep;24(9):1171-80. doi: 10.14670/HH-24.1171. Histol Histopathol. 2009. PMID: 19609864 Free PMC article. Review.
-
Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity.Trends Cell Biol. 2010 Oct;20(10):618-26. doi: 10.1016/j.tcb.2010.08.004. Trends Cell Biol. 2010. PMID: 20833047 Review.
Cited by
-
The clathrin adaptor AP-1A mediates basolateral polarity.Dev Cell. 2012 Apr 17;22(4):811-23. doi: 10.1016/j.devcel.2012.02.004. Dev Cell. 2012. PMID: 22516199 Free PMC article.
-
EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans.Mol Biol Cell. 2010 Aug 15;21(16):2930-43. doi: 10.1091/mbc.E10-02-0149. Epub 2010 Jun 23. Mol Biol Cell. 2010. PMID: 20573983 Free PMC article.
-
The role of endosomal-recycling in long-term potentiation.Cell Mol Life Sci. 2011 Jan;68(2):185-94. doi: 10.1007/s00018-010-0516-2. Epub 2010 Sep 6. Cell Mol Life Sci. 2011. PMID: 20820847 Free PMC article. Review.
-
Critical importance of RAB proteins for synaptic function.Small GTPases. 2018 Mar 4;9(1-2):145-157. doi: 10.1080/21541248.2016.1277001. Epub 2017 Apr 13. Small GTPases. 2018. PMID: 28146371 Free PMC article. Review.
-
Rgp1 contributes to craniofacial cartilage development and Rab8a-mediated collagen II secretion.Front Endocrinol (Lausanne). 2023 Feb 9;14:1120420. doi: 10.3389/fendo.2023.1120420. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843607 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

