Cholesterol loss enhances TrkB signaling in hippocampal neurons aging in vitro
- PMID: 18287532
- PMCID: PMC2366859
- DOI: 10.1091/mbc.e07-09-0897
Cholesterol loss enhances TrkB signaling in hippocampal neurons aging in vitro
Abstract
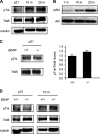
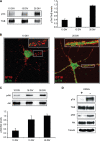
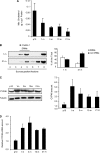
Binding of the neurotrophin brain-derived neurotrophic factor (BDNF) to the TrkB receptor is a major survival mechanism during embryonic development. In the aged brain, however, BDNF levels are low, suggesting that if TrkB is to play a role in survival at this stage additional mechanisms must have developed. We here show that TrkB activity is most robust in the hippocampus of 21-d-old BDNF-knockout mice as well as in old, wild-type, and BDNF heterozygous animals. Moreover, robust TrkB activity is evident in old but not young hippocampal neurons differentiating in vitro in the absence of any exogenous neurotrophin and also in neurons from BDNF -/- embryos. Age-associated increase in TrkB activity correlated with a mild yet progressive loss of cholesterol. This, in turn, correlated with increased expression of the cholesterol catabolic enzyme cholesterol 24-hydroxylase. Direct cause-effect, cholesterol loss-high TrkB activity was demonstrated by pharmacological means and by manipulating the levels of cholesterol 24-hydroxylase. Because reduced levels of cholesterol and increased expression of choleseterol-24-hydroxylase were also observed in the hippocampus of aged mice, changes in cellular cholesterol content may be used to modulate receptor activity strength in vivo, autonomously or as a way to complement the natural decay of neurotrophin production.
Figures





References
-
- Agerman K., Hjerling-Leffler J., Blanchard M. P., Scarfone E., Canlon B., Nosrat C., Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. - PubMed
-
- Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed
-
- Blum R., Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology. 2005;20:70–78. - PubMed
-
- Borroni B., Archetti S., Agosti C., Akkawi N., Brambilla C., Caimi L., Caltagirone C., Di Luca M., Padovani A. Intronic CYP46 polymorphism along with ApoE genotype in sporadic Alzheimer disease: from risk factors to disease modulators. Neurobiol. Aging. 2004;25:747–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

