Paralemmin-1, a modulator of filopodia induction is required for spine maturation
- PMID: 18287537
- PMCID: PMC2366842
- DOI: 10.1091/mbc.e07-08-0802
Paralemmin-1, a modulator of filopodia induction is required for spine maturation
Abstract
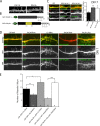
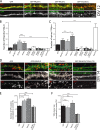
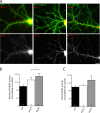
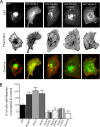
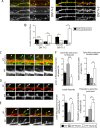
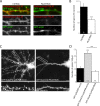
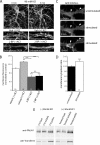
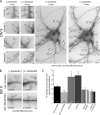
Dendritic filopodia are thought to participate in neuronal contact formation and development of dendritic spines; however, molecules that regulate filopodia extension and their maturation to spines remain largely unknown. Here we identify paralemmin-1 as a regulator of filopodia induction and spine maturation. Paralemmin-1 localizes to dendritic membranes, and its ability to induce filopodia and recruit synaptic elements to contact sites requires protein acylation. Effects of paralemmin-1 on synapse maturation are modulated by alternative splicing that regulates spine formation and recruitment of AMPA-type glutamate receptors. Paralemmin-1 enrichment at the plasma membrane is subject to rapid changes in neuronal excitability, and this process controls neuronal activity-driven effects on protrusion expansion. Knockdown of paralemmin-1 in developing neurons reduces the number of filopodia and spines formed and diminishes the effects of Shank1b on the transformation of existing filopodia into spines. Our study identifies a key role for paralemmin-1 in spine maturation through modulation of filopodia induction.
Figures
References
-
- Anderson R. G., Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
-
- Basile M., Lin R., Kabbani N., Karpa K., Kilimann M., Simpson I., Kester M. Paralemmin interacts with D3 dopamine receptors: implications for membrane localization and cAMP signaling. Arch. Biochem. Biophys. 2006;446:60–68. - PubMed
-
- Berzat A. C., Buss J. E., Chenette E. J., Weinbaum C. A., Shutes A., Der C. J., Minden A., Cox A. D. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem. 2005;280:33055–33065. - PubMed
-
- Bredt D. S., Nicoll R. A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
-
- Burwinkel B., Miglierini G., Jenne D. E., Gilbert D. J., Copeland N. G., Jenkins N. A., Ring H. Z., Francke U., Kilimann M. W. Structure of the human paralemmin gene (PALM), mapping to human chromosome 19p13.3 and mouse chromosome 10, and exclusion of coding mutations in grizzled, mocha, jittery, and hesitant mice. Genomics. 1998;49:462–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

