Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase
- PMID: 18287541
- PMCID: PMC2366846
- DOI: 10.1091/mbc.e06-12-1156
Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase
Abstract
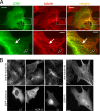
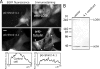
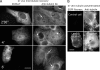
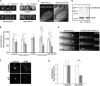
Interphase microtubules are organized into a radial array with centrosome in the center. This organization is a subject of cellular regulation that can be driven by protein phosphorylation. Only few protein kinases that regulate microtubule array in interphase cells have been described. Ste20-like protein kinase LOSK (SLK) was identified as a microtubule and centrosome-associated protein. In this study we have shown that the inhibition of LOSK activity by dominant-negative mutant K63R-DeltaT or by LOSK depletion with RNAi leads to unfocused microtubule arrangement. Microtubule disorganization is prominent in Vero, CV-1, and CHO-K1 cells but less distinct in HeLa cells. The effect is a result neither of microtubule stabilization nor of centrosome disruption. In cells with suppressed LOSK activity centrosomes are unable to anchor or to cap microtubules, though they keep nucleating microtubules. These centrosomes are depleted of dynactin. Vero cells overexpressing K63R-DeltaT have normal dynactin "comets" at microtubule ends and unaltered morphology of Golgi complex but are unable to polarize it at the wound edge. We conclude that protein kinase LOSK is required for radial microtubule organization and for the proper localization of Golgi complex in various cell types.
Figures




References
-
- Abe Y., Ohsugi M., Haraguchi K., Fujimoto J., Yamamoto T. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580:782–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

