Spatial heterogeneity of cortical receptive fields and its impact on multisensory interactions
- PMID: 18287544
- PMCID: PMC3637795
- DOI: 10.1152/jn.01386.2007
Spatial heterogeneity of cortical receptive fields and its impact on multisensory interactions
Abstract
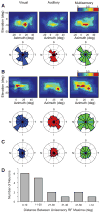
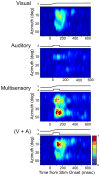
Investigations of multisensory processing at the level of the single neuron have illustrated the importance of the spatial and temporal relationship of the paired stimuli and their relative effectiveness in determining the product of the resultant interaction. Although these principles provide a good first-order description of the interactive process, they were derived by treating space, time, and effectiveness as independent factors. In the anterior ectosylvian sulcus (AES) of the cat, previous work hinted that the spatial receptive field (SRF) architecture of multisensory neurons might play an important role in multisensory processing due to differences in the vigor of responses to identical stimuli placed at different locations within the SRF. In this study the impact of SRF architecture on cortical multisensory processing was investigated using semichronic single-unit electrophysiological experiments targeting a multisensory domain of the cat AES. The visual and auditory SRFs of AES multisensory neurons exhibited striking response heterogeneity, with SRF architecture appearing to play a major role in the multisensory interactions. The deterministic role of SRF architecture was tightly coupled to the manner in which stimulus location modulated the responsiveness of the neuron. Thus multisensory stimulus combinations at weakly effective locations within the SRF resulted in large (often superadditive) response enhancements, whereas combinations at more effective spatial locations resulted in smaller (additive/subadditive) interactions. These results provide important insights into the spatial organization and processing capabilities of cortical multisensory neurons, features that may provide important clues as to the functional roles played by this area in spatially directed perceptual processes.
Figures




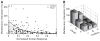
References
-
- Anastasio TJ, Patton PE, Belkacem-Boussaid K. Using Bayes’ rule to model multisensory enhancement in the superior colliculus. Neural Comput. 2000;12:1165–1187. - PubMed
-
- Avillac M, Deneve S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8:941–949. - PubMed
-
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J Cogn Neurosci. 2005;17:377–391. - PubMed
-
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nat Neurosci. 2004;7:1190–1192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

