The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis
- PMID: 18287564
- PMCID: PMC2390957
- DOI: 10.1681/ASN.2007040420
The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis
Abstract
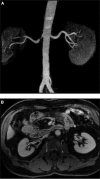
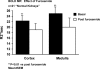
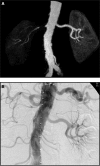
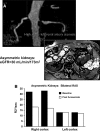
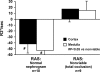
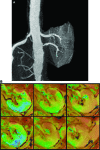
Vascular occlusive disease poses a threat to kidney viability, but whether the events leading to injury and eventual fibrosis actually entail reduced oxygenation and regional tissue ischemia is unknown. Answering this question has been difficult because of the lack of an adequate method to assess tissue oxygenation in humans. BOLD (blood oxygen-level-dependent) magnetic resonance imaging detects changes in tissue deoxyhemoglobin during maneuvers that affect oxygen consumption, therefore this technique was used to image and analyze cortical and medullary segments of 50 kidneys in 25 subjects undergoing magnetic resonance (MR) angiography to diagnose renal artery stenosis (RAS). Magnetic rate of relaxation (R2*) positively correlates with deoxyhemoglobin levels and was therefore used as a surrogate measure of tissue oxygenation. Furosemide was administered to examine the effect of inhibiting energy-dependent electrolyte transport on tissue oxygenation in subjects with renovascular disease. In 21 kidneys with normal nephrograms, administration of furosemide led to a 20% decrease in medullary R2* (P < 0.01) and an 11.2% decrease in cortical R2*. In normal-size kidneys downstream of high-grade renal arterial stenoses, R2* was elevated at baseline, but fell after furosemide. In contrast, atrophic kidneys beyond totally occluded renal arteries demonstrated low levels of R2* that did not change after furosemide. In kidneys with multiple arteries, localized renal artery stenoses produced focal elevations of R2*, suggesting areas of deoxyhemoglobin accumulation. These results suggest that BOLD MR coupled with a method to suppress tubular oxygen consumption can be used to evaluate regional tissue oxygenation in the human kidney affected by vascular occlusive disease.
Figures
References
-
- Garovic V, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation 112: 1362–1374, 2005 - PubMed
-
- Nielsen K, Rehling M, Henriksen JH: Renal vein oxygen saturation in renal artery stenosis. Clin Physiol 12: 179–184, 1992 - PubMed
-
- Textor SC, Lerman LO: Renal artery disease: Pathophysiology. In: Vascular Medicine: A Companion to Braunwald's Heart Disease, edited by Creager MA, Dzau VJ, Loscalzo J, Philadelphia, Saunders-Elsevier, 2006, pp 323–334
-
- Pohl MA: Renal artery stenosis, renal vascular hypertension and ischemic nephropathy. In: Diseases of the Kidney, Sixth Ed., edited by Schrier RW, Gottschalk CW, Boston, Little, Brown and Company, 1997, pp 1367–1423
-
- Alcazar JM, Rodicio JL: Ischemic Nephropathy: Clinical characteristics and treatment. Am J Kidney Dis 36: 883–893, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

