Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification
- PMID: 18288101
- PMCID: PMC3010853
- DOI: 10.1038/ki.2008.26
Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification
Abstract
Pyrophosphate is a potent inhibitor of medial vascular calcification where its level is controlled by hydrolysis via a tissue-nonspecific alkaline phosphatase (TNAP). We sought to determine if increased TNAP activity could explain the pyrophosphate deficiency and vascular calcification seen in renal failure. TNAP activity increased twofold in intact aortas and in aortic homogenates from rats made uremic by feeding adenine or by 5/6 nephrectomy. Immunoblotting showed an increase in protein abundance but there was no increase in TNAP mRNA assessed by quantitative polymerase chain reaction. Hydrolysis of pyrophosphate by rat aortic rings was inhibited about half by the nonspecific alkaline phosphatase inhibitor levamisole and was reduced about half in aortas from mice lacking TNAP. Hydrolysis was increased in aortic rings from uremic rats and all of this increase was inhibited by levamisole. An increase in TNAP activity and pyrophosphate hydrolysis also occurred when aortic rings from normal rats were incubated with uremic rat plasma. These results suggest that a circulating factor causes pyrophosphate deficiency by regulating TNAP activity and that vascular calcification in renal failure may result from the action of this factor. If proven by future studies, this mechanism will identify alkaline phosphatase as a potential therapeutic target.
Figures

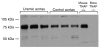

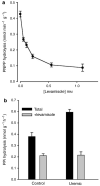


Comment in
-
Role for alkaline phosphatase as an inducer of vascular calcification in renal failure?Kidney Int. 2008 May;73(9):989-91. doi: 10.1038/ki.2008.104. Kidney Int. 2008. PMID: 18414436
References
-
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. New Eng J Med. 2000;342:1478–1483. - PubMed
-
- Memma HE, Oreopoulos DG, deVeber GA. Arterial calcifications in severe chronic renal disease and their relationship to dialysis treatment, renal transplant, and parathyroidectomy. Radiology. 1976;121:315–321. - PubMed
-
- O’Neill WC. The fallacy of the calcium phosphorus product. Kidney Int. 2007;72:792–796. - PubMed
-
- Everhart JE, Pettitt DJ, Knowler WC, et al. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

