Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis
- PMID: 18288313
- PMCID: PMC2235215
- DOI: 10.1289/ehp.10131
Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis
Abstract
Background: Chronic exposure to excess arsenic in drinking water has been strongly associated with increased risks of multiple cancers, diabetes, heart disease, and reproductive and developmental problems in humans. We previously demonstrated that As, a potent endocrine disruptor at low, environmentally relevant levels, alters steroid signaling at the level of receptor-mediated gene regulation for all five steroid receptors.
Objectives: The goal of this study was to determine whether As can also disrupt gene regulation via the retinoic acid (RA) receptor (RAR) and/or the thyroid hormone (TH) receptor (TR) and whether these effects are similar to previously observed effects on steroid regulation.
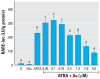
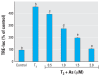
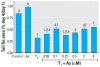
Methods and results: Human embryonic NT2 or rat pituitary GH3 cells were treated with 0.01-5 microM sodium arsenite for 24 hr, with or without RA or TH, respectively, to examine effects of As on receptor-mediated gene transcription. At low, noncytotoxic doses, As significantly altered RAR-dependent gene transcription of a transfected RAR response element-luciferase construct and the native RA-inducible cytochrome P450 CYP26A gene in NT2 cells. Likewise, low-dose As significantly altered expression of a transfected TR response element-luciferase construct and the endogenous TR-regulated type I deiodinase (DIO1) gene in a similar manner in GH3 cells. An amphibian ex vivo tail metamorphosis assay was used to examine whether endocrine disruption by low-dose As could have specific pathophysiologic consequences, because tail metamorphosis is tightly controlled by TH through TR. TH-dependent tail shrinkage was inhibited in a dose-dependent manner by 0.1- 4.0 microM As.
Conclusions: As had similar effects on RAR- and TR-mediated gene regulation as those previously observed for the steroid receptors, suggesting a common mechanism or action. Arsenic also profoundly affected a TR-dependent developmental process in a model animal system at very low concentrations. Because RAR and TH are critical for both normal human development and adult function and their dysregulation is associated with many disease processes, disruption of these hormone receptor-dependent processes by As is also potentially relevant to human developmental problems and disease risk.
Keywords: CYP26A; arsenic (As); deiodinase (DIO1); endocrine; retinoic acid (RA); steroid; thyroid (TH).
Figures


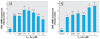


Similar articles
-
A multi-tiered, in vivo, quantitative assay suite for environmental disruptors of thyroid hormone signaling.Aquat Toxicol. 2017 Sep;190:1-10. doi: 10.1016/j.aquatox.2017.06.019. Epub 2017 Jun 21. Aquat Toxicol. 2017. PMID: 28662416 Free PMC article.
-
Evaluation of gene expression endpoints in the context of a Xenopus laevis metamorphosis-based bioassay to detect thyroid hormone disruptors.Aquat Toxicol. 2006 Jan 5;76(1):24-36. doi: 10.1016/j.aquatox.2005.09.003. Epub 2005 Nov 9. Aquat Toxicol. 2006. PMID: 16289343
-
Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development.Proc Natl Acad Sci U S A. 2000 Nov 21;97(24):13138-43. doi: 10.1073/pnas.260141297. Proc Natl Acad Sci U S A. 2000. PMID: 11078533 Free PMC article.
-
Autoinduction of nuclear receptor genes and its significance.J Steroid Biochem Mol Biol. 1993 Aug;46(2):105-19. doi: 10.1016/0960-0760(93)90286-6. J Steroid Biochem Mol Biol. 1993. PMID: 8664159 Review.
-
Thyroid hormone regulation of Xenopus laevis metamorphosis: functions of thyroid hormone receptors and roles of extracellular matrix remodeling.Wound Repair Regen. 1998 Jul-Aug;6(4):314-22. doi: 10.1046/j.1524-475x.1998.60407.x. Wound Repair Regen. 1998. PMID: 9824550 Review.
Cited by
-
Profiling microRNA expression in Atlantic killifish (Fundulus heteroclitus) gill and responses to arsenic and hyperosmotic stress.Aquat Toxicol. 2019 Jan;206:142-153. doi: 10.1016/j.aquatox.2018.11.009. Epub 2018 Nov 12. Aquat Toxicol. 2019. PMID: 30476744 Free PMC article.
-
Analysis of arsenic-modulated expression of hypothalamic estrogen receptor, thyroid receptor, and peroxisome proliferator-activated receptor gamma mRNA and simultaneous mitochondrial morphology and respiration rates in the mouse.PLoS One. 2024 May 16;19(5):e0303528. doi: 10.1371/journal.pone.0303528. eCollection 2024. PLoS One. 2024. PMID: 38753618 Free PMC article.
-
Permeation of roxarsone and its metabolites increases caco-2 cell proliferation.Adv Biol Chem. 2013 Aug;3(4):389-396. doi: 10.4236/abc.2013.34041. Adv Biol Chem. 2013. PMID: 25632371 Free PMC article.
-
Disruption of histone modification and CARM1 recruitment by arsenic represses transcription at glucocorticoid receptor-regulated promoters.PLoS One. 2009 Aug 26;4(8):e6766. doi: 10.1371/journal.pone.0006766. PLoS One. 2009. PMID: 19707557 Free PMC article.
-
Maternal arsenic exposure and DNA damage biomarkers, and the associations with birth outcomes in a general population from Taiwan.PLoS One. 2014 Feb 18;9(2):e86398. doi: 10.1371/journal.pone.0086398. eCollection 2014. PLoS One. 2014. PMID: 24558361 Free PMC article.
References
-
- Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. J Nutr. 2003;133(suppl 1):1536S–1538S. - PubMed
-
- Allen BM. The results of extirpation of the anterior lobe of the hypophysis and the thyroid of Rana pipiens larvae. Science. 1916;44:755–758. - PubMed
-
- Allen T, Rana SV. Oxidative stress by inorganic arsenic: modulation by thyroid hormones in rat. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

