Immobilization of heparin: approaches and applications
- PMID: 18289079
- PMCID: PMC4117378
- DOI: 10.2174/156802608783378891
Immobilization of heparin: approaches and applications
Abstract
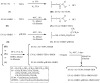
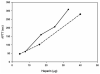
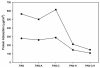
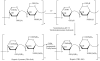
Heparin, an anticoagulant, has been used in many forms to treat various diseases. These forms include soluble heparin and heparin immobilized to supporting matrices by physical adsorption, by covalent chemical methods and by photochemical attachment. These immobilization methods often require the use of spacers or linkers. This review examines and compares various techniques that have been used for the immobilization of heparin as well as applications of these immobilized heparins. In the applications reviewed, immobilized heparin is compared with soluble heparin for efficient and versatile use in each of the various applications.
Figures


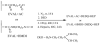

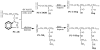









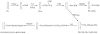
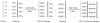



Similar articles
-
Modified heparin inhibits P-selectin-mediated cell adhesion of human colon carcinoma cells to immobilized platelets under dynamic flow conditions.J Biol Chem. 2004 Jul 9;279(28):29202-10. doi: 10.1074/jbc.M312951200. Epub 2004 May 7. J Biol Chem. 2004. PMID: 15133030
-
Synthesis and evaluation of heparin immobilized "side-on" to polystyrene microspheres coated with end-group activated polyethylene oxide.Int J Biol Macromol. 2010 Aug 1;47(2):98-103. doi: 10.1016/j.ijbiomac.2010.05.015. Epub 2010 May 26. Int J Biol Macromol. 2010. PMID: 20621768
-
New methods for surface modification and covalent attachment of heparin.Med Device Technol. 1998 Jan-Feb;9(1):24-7. Med Device Technol. 1998. PMID: 10176141
-
[Review of heparin immobilization technique].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007 Apr;24(2):466-9. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007. PMID: 17591284 Review. Chinese.
-
Heparin-Mimicking Polymers: Synthesis and Biological Applications.Biomacromolecules. 2016 Nov 14;17(11):3417-3440. doi: 10.1021/acs.biomac.6b01147. Epub 2016 Oct 14. Biomacromolecules. 2016. PMID: 27739666 Free PMC article. Review.
Cited by
-
Grafting Strategies of Oxidation-Prone Coiled-Coil Peptides for Protein Capture in Bioassays: Impact of Orientation and the Oxidation State.ACS Omega. 2023 Jul 28;8(31):28301-28313. doi: 10.1021/acsomega.3c02172. eCollection 2023 Aug 8. ACS Omega. 2023. PMID: 37576632 Free PMC article.
-
Heparinized Polyurethane Surface Via a One-Step Photografting Method.Molecules. 2019 Feb 20;24(4):758. doi: 10.3390/molecules24040758. Molecules. 2019. PMID: 30791534 Free PMC article.
-
Xanthan-Based Materials as a Platform for Heparin Delivery.Molecules. 2023 Mar 18;28(6):2757. doi: 10.3390/molecules28062757. Molecules. 2023. PMID: 36985729 Free PMC article.
-
Ex vivo evaluation of blood coagulation on endothelial glycocalyx-inspired surfaces using thromboelastography.In Vitro Model. 2021 Oct 29;1(1):59-71. doi: 10.1007/s44164-021-00001-w. eCollection 2022 Feb. In Vitro Model. 2021. PMID: 39872977 Free PMC article.
-
Advanced strategies to thwart foreign body response to implantable devices.Bioeng Transl Med. 2022 Mar 2;7(3):e10300. doi: 10.1002/btm2.10300. eCollection 2022 Sep. Bioeng Transl Med. 2022. PMID: 36176611 Free PMC article. Review.
References
-
- Rydberg E, Westfall MJ, Nicholas RA. Low Molecular weight heparin in preventing and treating DVT. Am. Fam. Physician. 1999:1607. - PubMed
-
- Sanchez J, Elgue G, Riesenfeld J, Olsson P. Studies of adsorption, activation, and inhibition of factor XII on immobilized heparin. Thromb. Res. 1998;89:41–50. - PubMed
-
- Jackson RL, Busch SJ, Cardin ADW. Physiol. Rev. 1991;71:481–539. - PubMed
-
- Danishefsky I, Tzeng F, Ahrens M, Klien S. Thromb. Res. 1976;8:131–140. - PubMed
-
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

