Integrin regulation of cytoplasmic calcium in excitatory neurons depends upon glutamate receptors and release from intracellular stores
- PMID: 18289871
- PMCID: PMC2396149
- DOI: 10.1016/j.mcn.2008.01.001
Integrin regulation of cytoplasmic calcium in excitatory neurons depends upon glutamate receptors and release from intracellular stores
Abstract
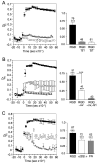
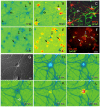
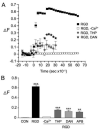
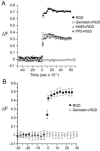
Integrins regulate cytoplasmic calcium levels ([Ca(2+)]i) in various cell types but information on activities in neurons is limited. The issue is of current interest because of the evidence that both integrins and changes in [Ca(2+)]i are required for Long-Term Potentiation. Accordingly, the present studies evaluated integrin ligand effects in cortical neurons. Integrin ligands or alpha5beta1 integrin activating antisera rapidly increased [Ca(2+)]i with effects greater in glutamatergic than GABAergic neurons, absent in astroglia, and blocked by beta1 integrin neutralizing antisera and the tyrosine kinase antagonist genistein. Increases depended upon extracellular calcium and intracellular store release. Ligand-induced effects were reduced by voltage-sensitive calcium channel and NMDA receptor antagonists, but blocked by tetrodotoxin or AMPA receptor antagonists. These results indicate that integrin ligation triggers AMPA receptor/depolarization-dependent calcium influx followed by intracellular store release and suggest the possibility that integrin modulation of activity-induced changes in [Ca(2+)]i contributes importantly to lasting synaptic plasticity in forebrain neurons.
Figures



Similar articles
-
Glutamate-induced increases in intracellular Ca2+ in cultured rat neocortical neurons.Neuroreport. 2002 Jun 12;13(8):1051-6. doi: 10.1097/00001756-200206120-00015. Neuroreport. 2002. PMID: 12060807
-
AMPA receptor stimulation increases alpha5beta1 integrin surface expression, adhesive function and signaling.J Neurochem. 2005 Jul;94(2):531-46. doi: 10.1111/j.1471-4159.2005.03203.x. J Neurochem. 2005. PMID: 16000124 Free PMC article.
-
Neuregulin beta1 enhances peak glutamate-induced intracellular calcium levels through endoplasmic reticulum calcium release in cultured hippocampal neurons.Can J Physiol Pharmacol. 2009 Oct;87(10):883-91. doi: 10.1139/Y09-082. Can J Physiol Pharmacol. 2009. PMID: 20052014
-
Neurotoxicity and excitatory amino acid antagonists.Neurotoxicology. 1994 Fall;15(3):477-81. Neurotoxicology. 1994. PMID: 7854581 Review.
-
Excitatory amino acid-mediated cytotoxicity and calcium homeostasis in cultured neurons.J Neurochem. 1993 Apr;60(4):1202-11. doi: 10.1111/j.1471-4159.1993.tb03278.x. J Neurochem. 1993. PMID: 8455022 Review.
Cited by
-
NX210c Peptide Promotes Glutamatergic Receptor-Mediated Synaptic Transmission and Signaling in the Mouse Central Nervous System.Int J Mol Sci. 2022 Aug 9;23(16):8867. doi: 10.3390/ijms23168867. Int J Mol Sci. 2022. PMID: 36012124 Free PMC article.
-
Integrin and GPCR Crosstalk in the Regulation of ASM Contraction Signaling in Asthma.J Allergy (Cairo). 2012;2012:341282. doi: 10.1155/2012/341282. Epub 2012 Sep 29. J Allergy (Cairo). 2012. PMID: 23056062 Free PMC article.
-
Custom-engineered hydrogels for delivery of human iPSC-derived neurons into the injured cervical spinal cord.Biomaterials. 2024 Mar;305:122400. doi: 10.1016/j.biomaterials.2023.122400. Epub 2023 Nov 17. Biomaterials. 2024. PMID: 38134472 Free PMC article.
-
Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation.J Neurosci. 2012 Sep 12;32(37):12854-61. doi: 10.1523/JNEUROSCI.2024-12.2012. J Neurosci. 2012. PMID: 22973009 Free PMC article.
-
A possible role for integrin signaling in diffuse axonal injury.PLoS One. 2011;6(7):e22899. doi: 10.1371/journal.pone.0022899. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799943 Free PMC article.
References
-
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. - PubMed
-
- Bernard-Trifilo JA, Kramár EA, Torp R, Lin C-Y, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93:834–849. - PubMed
-
- Bhattacharya S, Ying X, Fu C, Patel R, Kuebler W, Greenberg S, Battacharya J. αvß3 integrin induces tyrosine phosphorylation-dependent Ca++ influx in pulmonary endothelial cells. Circ Res. 2000;86:456–462. - PubMed
-
- Bi X, Lynch G, Zhou J, Gall CM. Polarized distribution of α5 integrin in the dendrites of hippocampal and cortical neurons. J. Comp. Neurol. 2001;435:184–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

