A vascular anatomical network model of the spatio-temporal response to brain activation
- PMID: 18289880
- PMCID: PMC2577617
- DOI: 10.1016/j.neuroimage.2007.12.061
A vascular anatomical network model of the spatio-temporal response to brain activation
Abstract
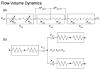
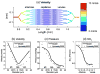
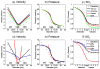
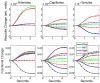
Neuronal activity-induced changes in vascular tone and oxygen consumption result in a dynamic evolution of blood flow, volume, and oxygenation. Functional neuroimaging techniques, such as functional magnetic resonance imaging, optical imaging, and PET, provide indirect measures of the neural-induced vascular dynamics driving the blood parameters. Models connecting changes in vascular tone and oxygen consumption to observed changes in the blood parameters are needed to guide more quantitative physiological interpretation of these functional neuroimaging modalities. Effective lumped-parameter vascular balloon and Windkessel models have been developed for this purpose, but the lumping of the complex vascular network into a series of arterioles, capillaries, and venules allows only qualitative interpretation. We have therefore developed a parallel vascular anatomical network (VAN) model based on microscopically measurable properties to improve quantitative interpretation of the vascular response. The model, derived from measured physical properties, predicts baseline blood pressure and oxygen saturation distributions and dynamic responses consistent with literature. Furthermore, the VAN model allows investigation of spatial features of the dynamic vascular and oxygen response to neuronal activity. We find that a passive surround negative vascular response ("negative BOLD") is predicted, but that it underestimates recently observed surround negativity suggesting that additional active surround vasoconstriction is required to explain the experimental data.
Figures





References
-
- Albeck MJ, Borgesen SE, Gjerris F, Schmidt JF, Sorensen PS. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J Neurosurg. 1991;74:597–600. - PubMed
-
- Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T. MR-intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology. 2000;217:877–885. - PubMed
-
- Ances BM, Wilson DF, Greenberg JH, Detre JA. Dynamic changes in cerebral blood flow, O2 tension, and calculated cerebral metabolic rate of O2 during functional activation using oxygen phosphorescence quenching. J Cereb Blood Flow Metab. 2001;21:511–516. - PubMed
-
- Behzadi Y, Liu TT. An arteriolar compliance model of the cerebral blood flow response to neural stimulus. Neuroimage. 2005;25:1100–1111. - PubMed
-
- Boas DA, Strangman G, Culver JP, Hoge RD, Jasdzewski G, Poldrack RA, Rosen BR, Mandeville JB. Can the cerebral metabolic rate of oxygen be estimated with near-infrared spectroscopy? Phys Med Biol. 2003;48:2405–2418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

