Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma
- PMID: 18291119
- PMCID: PMC2374742
- DOI: 10.1016/j.freeradbiomed.2008.01.022
Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma
Abstract
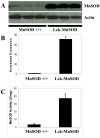
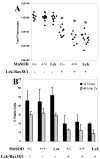
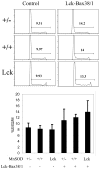
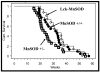
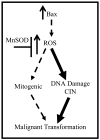
Increased reactive oxygen species (ROS) such as superoxide have been implicated as causal elements of oncogenesis. A variety of cancers have displayed changes in steady-state levels of key antioxidant enzymes, with the mitochondrial form of superoxide dismutase (MnSOD) being commonly implicated. Increasing MnSOD expression suppresses the malignant phenotype in various cancer cell lines and suppresses tumor formation in xenograft and transgenic mouse models. We examined the impact of MnSOD expression in the development of T cell lymphoma in mice expressing proapoptotic Bax. Lck-Bax38/1 transgenic mice were crossed to mice overexpressing MnSOD (Lck-MnSOD) as well as MnSOD+/- mice. The effects of MnSOD on apoptosis, cell cycle, chromosomal instability (CIN), and lymphoma development were determined. The apoptotic and cell cycle phenotypes observed in thymocytes from control and Bax transgenic mice were unaffected by variations in MnSOD levels. Remarkably, increased gene dosage of MnSOD significantly decreased aneuploidy in premalignant thymocytes as well as the onset of tumor formation in Lck-Bax38/1 mice. The observed effects of MnSOD support a role for ROS in CIN and tumor formation in this mouse model of T cell lymphoma.
Figures




Similar articles
-
Chromosomal instability and supernumerary centrosomes represent precursor defects in a mouse model of T-cell lymphoma.Cancer Res. 2007 Sep 1;67(17):8081-8. doi: 10.1158/0008-5472.CAN-07-1666. Cancer Res. 2007. PMID: 17804719
-
Mimic of manganese superoxide dismutase to induce apoptosis of human non-Hodgkin lymphoma Raji cells through mitochondrial pathways.Int Immunopharmacol. 2012 Dec;14(4):620-8. doi: 10.1016/j.intimp.2012.09.019. Epub 2012 Oct 12. Int Immunopharmacol. 2012. PMID: 23069381
-
Lymphoma development in Bax transgenic mice is inhibited by Bcl-2 and associated with chromosomal instability.Cell Death Differ. 2003 Jun;10(6):740-8. doi: 10.1038/sj.cdd.4401233. Cell Death Differ. 2003. PMID: 12761582
-
Managing odds in stem cells: insights into the role of mitochondrial antioxidant enzyme MnSOD.Free Radic Res. 2016;50(5):570-84. doi: 10.3109/10715762.2016.1155708. Free Radic Res. 2016. PMID: 26899340 Review.
-
Invited review: manganese superoxide dismutase in disease.Free Radic Res. 2001 Apr;34(4):325-36. doi: 10.1080/10715760100300281. Free Radic Res. 2001. PMID: 11328670 Review.
Cited by
-
The functional role of MnSOD as a biomarker of human diseases and therapeutic potential of a new isoform of a human recombinant MnSOD.Biomed Res Int. 2014;2014:476789. doi: 10.1155/2014/476789. Epub 2014 Jan 6. Biomed Res Int. 2014. PMID: 24511533 Free PMC article.
-
Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species.Oncol Lett. 2018 Sep;16(3):2771-2776. doi: 10.3892/ol.2018.9023. Epub 2018 Jun 26. Oncol Lett. 2018. PMID: 30127861 Free PMC article. Review.
-
Tumorigenic polyploid cells contain elevated ROS and ARE selectively targeted by antioxidant treatment.J Cell Physiol. 2012 Feb;227(2):801-12. doi: 10.1002/jcp.22793. J Cell Physiol. 2012. PMID: 21503880 Free PMC article.
-
Low-dose radiation-induced enhancement of thymic lymphomagenesis in Lck-Bax mice is dependent on LET and gender.Radiat Res. 2013 Aug;180(2):156-65. doi: 10.1667/RR3293.1. Epub 2013 Jul 2. Radiat Res. 2013. PMID: 23819597 Free PMC article.
-
Hydrogen peroxide signaling is required for glucocorticoid-induced apoptosis in lymphoma cells.Free Radic Biol Med. 2011 Dec 1;51(11):2048-59. doi: 10.1016/j.freeradbiomed.2011.09.002. Epub 2011 Sep 10. Free Radic Biol Med. 2011. PMID: 21964507 Free PMC article.
References
-
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. - PubMed
-
- Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M. Somatic frameshift mutations in the bax gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. - PubMed
-
- Meijerink JPP, Mensink EJBM, Wang K, Sedlak TW, Sloetjes AW, Dewitte T, Waksman G, Korsmeyer SJ. Hematopoietic malignancies demonstrate loss-of-function mutations of. BAX Blood. 1998;91:2991–2997. - PubMed
-
- Luke JJ, Van de Wetering CI, Knudson CM. Lymphoma development in Bax transgenic mice is inhibited by Bcl-2 and associated with chromosomal instability. Cell Death Differ. 2003;10:740–748. - PubMed
-
- de la Coste A, Mignon A, Fabre M, Gilbert E, Porteu A, Van Dyke T, Kahn A, Perret C. Paradoxical inhibition of c-myc-induced carcinogenesis by Bcl-2 in transgenic mice. Cancer Res. 1999;59:5017–5022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

