Single-molecule atomic force spectroscopy reveals that DnaD forms scaffolds and enhances duplex melting
- PMID: 18291414
- PMCID: PMC3033579
- DOI: 10.1016/j.jmb.2008.01.067
Single-molecule atomic force spectroscopy reveals that DnaD forms scaffolds and enhances duplex melting
Abstract
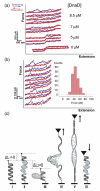
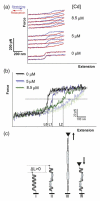
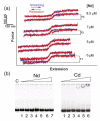
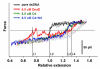
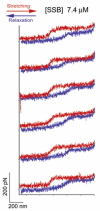
The Bacillus subtilis DnaD is an essential DNA-binding protein implicated in replication and DNA remodeling. Using single-molecule atomic force spectroscopy, we have studied the interaction of DnaD and its domains with DNA. Our data reveal that binding of DnaD to immobilized single molecules of duplex DNA causes a marked reduction in the 'end-to-end' distance of the DNA in a concentration-dependent manner, consistent with previously reported DnaD-induced looping by scaffold formation. Native DnaD enhances partial melting of the DNA strands. The C-terminal domain (Cd) of DnaD binds to DNA and enhances partial duplex melting but does not cause DNA looping. The Cd-mediated melting is not as efficient as that caused by native DnaD. The N-terminal domain (Nd) does not affect significantly the DNA. A mixture of Nd and Cd fails to recreate the DNA looping effect of native DnaD but produces exactly the same effects as Cd on its own, consistent with the previously reported failure of the separated domains to form DNA-interacting scaffolds.
Figures

References
-
- Smith SB, Finzi L, Bustamante C. Direct mechanical measurements of the elasticity of single molecules by using magnetic beads. Science. 1992;258:1122–1126. - PubMed
-
- Bensimon D. Force: a new structural control parameter? Structure. 1996;4:885–889. - PubMed
-
- Strick TR, Allemand J-F, Bensimon D, Bensimon A, Croquette V. The elasticity of a single supercoiled DNA molecule. Science. 1996;271:1835–1837. - PubMed
-
- Strick TR, Allemand JF, Bensimon D, Croquette V. Stress-induced structural transition in DNA and proteins. Annu. Rev. Biophys. Biomol. Struct. 2000;29:523–543. - PubMed
-
- Bustamante C, Bryant Z, Smith SB. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

