Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function
- PMID: 18292131
- PMCID: PMC2465204
- DOI: 10.1113/jphysiol.2007.147967
Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function
Abstract
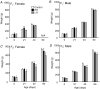
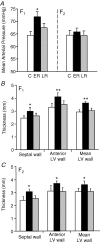
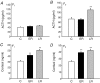
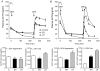
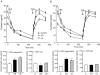
The perinatal environment is a powerful determinant of risk for developing disease in later life. Here, we have shown that maternal undernutrition causes dramatic changes in heart structure and hypothalamo-pituitary-adrenal (HPA) function across two generations. Pregnant guinea pigs were fed 70% of normal intake from gestational days 1-35 (early restriction; ER), or 36-70 (late restriction; LR). Female offspring (F(1)) were mated and fed ad libitum to create second generation (F(2)) offspring. Heart morphology, blood pressure, baroreceptor and HPA function were assessed in male F(1) and F(2) offspring. ER(F1) males exhibited elevated blood pressure, increased left ventricular (LV) wall thickness and LV mass. These LV effects were maintained in the ER(F2) offspring. Maternal undernutrition increased basal cortisol and altered HPA responsiveness to challenge in both generations; effects were greatest in LR groups. In conclusion, moderate maternal undernutrition profoundly modifies heart structure and HPA function in adult male offspring for two generations.
Figures
References
-
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. - PubMed
-
- Bendeck MP, Keeley FW, Langille BL. Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol Heart Circ Physiol. 1994;267:H2268–H2279. - PubMed
-
- Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2, 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. - PubMed
-
- Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–439. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

