Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition
- PMID: 18292227
- PMCID: PMC2265168
- DOI: 10.1073/pnas.0712140105
Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition
Abstract
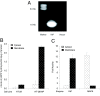
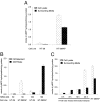
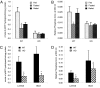
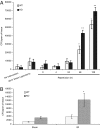
Under conditions of starvation and disease, the gut barrier becomes impaired, and trophic feeding to prevent gut mucosal atrophy has become a standard treatment of critically ill patients. However, the mechanisms responsible for the beneficial effects of enteral nutrition have remained a mystery. Using in vitro and in vivo models, we demonstrate that the brush-border enzyme, intestinal alkaline phosphatase (IAP), has the ability to detoxify lipopolysaccharide and prevent bacterial invasion across the gut mucosal barrier. IAP expression and function are lost with starvation and maintained by enteral feeding. It is likely that the IAP silencing that occurs during starvation is a key component of the gut mucosal barrier dysfunction seen in critically ill patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416. - PubMed
-
- Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59–67. - PubMed
-
- Fink MP. Intestinal epithelial hyperpermeability: Update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. - PubMed
-
- Fink MP, Delude RL. Epithelial barrier dysfunction: A unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

