Curcumin prevents and reverses murine cardiac hypertrophy
- PMID: 18292803
- PMCID: PMC2248327
- DOI: 10.1172/JCI32865
Curcumin prevents and reverses murine cardiac hypertrophy
Retraction in
-
Curcumin prevents and reverses murine cardiac hypertrophy.J Clin Invest. 2009 Jul;119(7):2113. doi: 10.1172/jci32865r1. J Clin Invest. 2009. PMID: 19603550 Free PMC article. No abstract available.
Abstract
Chromatin remodeling, particularly histone acetylation, plays a critical role in the progression of pathological cardiac hypertrophy and heart failure. We hypothesized that curcumin, a natural polyphenolic compound abundant in the spice turmeric and a known suppressor of histone acetylation, would suppress cardiac hypertrophy through the disruption of p300 histone acetyltransferase-dependent (p300-HAT-dependent) transcriptional activation. We tested this hypothesis using primary cultured rat cardiac myocytes and fibroblasts as well as two well-established mouse models of cardiac hypertrophy. Curcumin blocked phenylephrin-induced (PE-induced) cardiac hypertrophy in vitro in a dose-dependent manner. Furthermore, curcumin both prevented and reversed mouse cardiac hypertrophy induced by aortic banding (AB) and PE infusion, as assessed by heart weight/BW and lung weight/BW ratios, echocardiographic parameters, and gene expression of hypertrophic markers. Further investigation demonstrated that curcumin abrogated histone acetylation, GATA4 acetylation, and DNA-binding activity through blocking p300-HAT activity. Curcumin also blocked AB-induced inflammation and fibrosis through disrupting p300-HAT-dependent signaling pathways. Our results indicate that curcumin has the potential to protect against cardiac hypertrophy, inflammation, and fibrosis through suppression of p300-HAT activity and downstream GATA4, NF-kappaB, and TGF-beta-Smad signaling pathways.
Figures

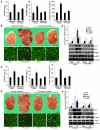

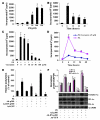



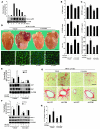
Comment in
-
Currying favor for the heart.J Clin Invest. 2008 Mar;118(3):850-2. doi: 10.1172/JCI34650. J Clin Invest. 2008. PMID: 18292806 Free PMC article.
References
-
- Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat. Rev. Mol. Cell Biol. 2006;7:589–600. - PubMed
-
- Chen F., et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. - PubMed
-
- Backs J., Olson E.N. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 2006;98:15–24. - PubMed
-
- Clayton A.L., Hazzalin C.A., Mahadevan L.C. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

