Growth hormone enhances thymic function in HIV-1-infected adults
- PMID: 18292808
- PMCID: PMC2248326
- DOI: 10.1172/JCI32830
Growth hormone enhances thymic function in HIV-1-infected adults
Abstract
Growth hormone (GH) is an underappreciated but important regulator of T cell development that can reverse age-related declines in thymopoiesis in rodents. Here, we report findings of a prospective randomized study examining the effects of GH on the immune system of HIV-1-infected adults. GH treatment was associated with increased thymic mass. In addition, GH treatment enhanced thymic output, as measured by both the frequency of T cell receptor rearrangement excision circles in circulating T cells and the numbers of circulating naive and total CD4(+) T cells. These findings provide compelling evidence that GH induces de novo T cell production and may, accordingly, facilitate CD4(+) T cell recovery in HIV-1-infected adults. Further, these randomized, prospective data have shown that thymic involution can be pharmacologically reversed in humans, suggesting that immune-based therapies could be used to enhance thymopoiesis in immunodeficient individuals.
Figures


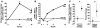


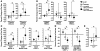
Comment in
-
Growth hormone resurrects adult human thymus during HIV-1 infection.J Clin Invest. 2008 Mar;118(3):844-7. doi: 10.1172/JCI35112. J Clin Invest. 2008. PMID: 18292816 Free PMC article.
-
Effects of growth hormone in enhancing thymic regrowth and T-cell reconstitution.Expert Rev Clin Immunol. 2008 Jul;4(4):433-9. doi: 10.1586/1744666X.4.4.433. Expert Rev Clin Immunol. 2008. PMID: 20477572
References
-
- Connors M., et al. HIV induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 1997;3:533–540. - PubMed
-
- Teixeira L., et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. - PubMed
-
- Lecossier D., Bouchonnet F., Schneider P., Clavel F., Hance A.J. Discordant increases in CD4+ T cells in human immunodeficiency virus-infected patients experiencing virologic treatment failure: role of changes in thymic output and T cell death. J. Infect. Dis. 2001;183:1009–1016. - PubMed
-
- Kaufmann G.R., et al. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 2002;16:359–367. - PubMed
-
- Kaufmann G.R., et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch. Intern. Med. 2003;163:2187–2195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI40312/AI/NIAID NIH HHS/United States
- AI47062/AI/NIAID NIH HHS/United States
- DP1 OD000329/OD/NIH HHS/United States
- M01 RR00083/RR/NCRR NIH HHS/United States
- AI062457/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- AI01597/AI/NIAID NIH HHS/United States
- DPI OD00329/OD/NIH HHS/United States
- R21 AI062457/AI/NIAID NIH HHS/United States
- P30 MH59037/MH/NIMH NIH HHS/United States
- R37 AI040312/AI/NIAID NIH HHS/United States
- R01 AI040312/AI/NIAID NIH HHS/United States
- AI43864/AI/NIAID NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- U01 AI043864/AI/NIAID NIH HHS/United States
- R01 AI047062/AI/NIAID NIH HHS/United States
- RR00083-44/RR/NCRR NIH HHS/United States
- R01 AI043864/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

