Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells
- PMID: 18292810
- PMCID: PMC2248804
- DOI: 10.1172/JCI34409
Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells
Erratum in
- J Clin Invest. 2008 Apr;118(4):1584. Abdul, Tawab [corrected to Tawab, Abdul]
Abstract
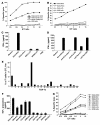
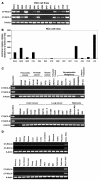
Transplanted donor lymphocytes infused during hematopoietic stem cell transplantation (HSCT) have been shown to cure patients with hematological malignancies. However, less is known about the effects of HSCT on metastatic solid tumors. Thus, a better understanding of the immune cells and their target antigens that mediate tumor regression is urgently needed to develop more effective HSCT approaches for solid tumors. Here we report regression of metastatic renal cell carcinoma (RCC) in patients following nonmyeloablative HSCT consistent with a graft-versus-tumor effect. We detected RCC-reactive donor-derived CD8(+) T cells in the blood of patients following nonmyeloablative HSCT. Using cDNA expression cloning, we identified a 10-mer peptide (CT-RCC-1) as a target antigen of RCC-specific CD8(+) T cells. The genes encoding this antigen were found to be derived from human endogenous retrovirus (HERV) type E and were expressed in RCC cell lines and fresh RCC tissue but not in normal kidney or other tissues. We believe this to be the first solid tumor antigen identified using allogeneic T cells from a patient undergoing HSCT. These data suggest that HERV-E is activated in RCC and that it encodes an overexpressed immunogenic antigen, therefore providing a potential target for cellular immunity.
Figures





References
-
- Childs R., et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. - PubMed
-
- Bregni M., Ueno N.T., Childs R. The second international meeting on allogeneic transplantation in solid tumors. Bone Marrow Transplant. 2006;38:527–537. - PubMed
-
- Sprangers B., Van Wijmeersch B., Fevery S., Waer M., Billiau A.D. Experimental and clinical approaches for optimization of the graft-versus-leukemia effect. Nat. Clin. Pract. Oncol. 2007;4:404–414. - PubMed
-
- Tykodi S.S., et al. Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin. Cancer Res. 2004;10:7799–7811. - PubMed
-
- Childs R., et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N. Engl. J. Med. 2000;343:750–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

