ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver
- PMID: 18292813
- PMCID: PMC2248330
- DOI: 10.1172/JCI34314
ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver
Abstract
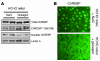
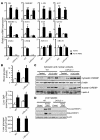
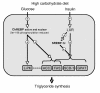
The transcription factor carbohydrate-responsive element-binding protein (ChREBP) has emerged as a central regulator of lipid synthesis in liver because it is required for glucose-induced expression of the glycolytic enzyme liver-pyruvate kinase (L-PK) and acts in synergy with SREBP to induce lipogenic genes such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). Liver X receptors (LXRs) are also important regulators of the lipogenic pathway, and the recent finding that ChREBP is a direct target of LXRs and that glucose itself can bind and activate LXRs prompted us to study the role of LXRs in the induction of glucose-regulated genes in liver. Using an LXR agonist in wild-type mice, we found that LXR stimulation did not promote ChREBP phosphorylation or nuclear localization in the absence of an increased intrahepatic glucose flux. Furthermore, the induction of ChREBP, L-PK, and ACC by glucose or high-carbohydrate diet was similar in LXRalpha/beta knockout compared with wild-type mice, suggesting that the activation of these genes by glucose occurs by an LXR-independent mechanism. We used fluorescence resonance energy transfer analysis to demonstrate that glucose failed to promote the interaction of LXRalpha/beta with specific cofactors. Finally, siRNA silencing of ChREBP in LXRalpha/beta knockout hepatocytes abrogated glucose-induced expression of L-PK and ACC, further demonstrating the central role of ChREBP in glucose signaling. Taken together, our results demonstrate that glucose is required for ChREBP functional activity and that LXRs are not necessary for the induction of glucose-regulated genes in liver.
Figures


Comment in
-
Hepatic glucose sensing: does flux matter?J Clin Invest. 2008 Mar;118(3):841-4. doi: 10.1172/JCI35137. J Clin Invest. 2008. PMID: 18292804 Free PMC article.
References
-
- Decaux J.F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J. Biol. Chem. 1989;264:11584–11590. - PubMed
-
- Dentin R., Girard J., Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. - PubMed
-
- Dentin R., et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

