A critical role for extracellular protein disulfide isomerase during thrombus formation in mice
- PMID: 18292814
- PMCID: PMC2248441
- DOI: 10.1172/JCI34134
A critical role for extracellular protein disulfide isomerase during thrombus formation in mice
Abstract
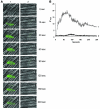
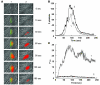
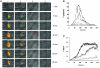
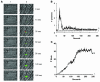
Thiol isomerases, including protein disulfide isomerase (PDI), catalyze disulfide oxidation, reduction, and isomerization, thereby playing an important role in protein synthesis. To determine whether extracellular PDI mediates thrombus formation in an animal model, PDI expression, platelet accumulation, and fibrin generation were monitored in the blood vessels of mice by intravital fluorescence microscopy following laser-induced arteriolar injury. A time-dependent increase in PDI was observed in murine thrombi following injury. Infusion of the PDI inhibitor bacitracin or a blocking monoclonal antibody against PDI inhibited platelet thrombus formation and fibrin generation. Fibrin deposition is normal in mice lacking the G protein-coupled platelet receptor Par4, although there is no stable accumulation of platelets. Infusion of monoclonal antibodies against PDI into the circulation of Par4(-/-) mice prior to vessel wall injury inhibited fibrin generation. These results indicate that PDI is required in vivo in mice for both fibrin generation and platelet thrombus formation.
Figures


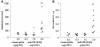
References
-
- Chen V.M., Hogg P.J. Allosteric disulfide bonds in thrombosis and thrombolysis. J. Thromb. Haemost. 2006;4:2533–2541. - PubMed
-
- Chen K., Lin Y., Detwiler T.C. Protein disulfide isomerase activity is released by activated platelets. Blood. 1992;79:2226–2228. - PubMed
-
- Essex D.W., Chen K., Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168–2173. - PubMed
-
- Thomas G., Skrinska V.A., Lucas F.V. The influence of glutathione and other thiols on human platelet aggregation. Thromb. Res. 1986;44:859–866. - PubMed
-
- Essex D.W., Li M. Redox control of platelet aggregation. Biochemistry. 2003;42:129–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

