Complex rectification of Müller cell Kir currents
- PMID: 18293411
- PMCID: PMC9930535
- DOI: 10.1002/glia.20652
Complex rectification of Müller cell Kir currents
Abstract
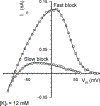
Although Kir4.1 channels are the major inwardly rectifying channels in glial cells and are widely accepted to support K+- and glutamate-uptake in the nervous system, the properties of Kir4.1 channels during vital changes of K+ and polyamines remain poorly understood. Therefore, the present study examined the voltage-dependence of K+ conductance with varying physiological and pathophysiological external [K+] and intrapipette spermine ([SP]) concentrations in Müller glial cells and in tsA201 cells expressing recombinant Kir4.1 channels. Two different types of [SP] block were characterized: "fast" and "slow." Fast block was steeply voltage-dependent, with only a low sensitivity to spermine and strong dependence on extracellular potassium concentration, [K+]o. Slow block had a strong voltage sensitivity that begins closer to resting membrane potential and was essentially [K+]o-independent, but with a higher spermine- and [K+]i-sensitivity. Using a modified Woodhull model and fitting i/V curves from whole cell recordings, we have calculated free [SP](in) in Müller glial cells as 0.81 +/- 0.24 mM. This is much higher than has been estimated previously in neurons. Biphasic block properties underlie a significantly varying extent of rectification with [K+] and [SP]. While confirming similar properties of glial Kir and recombinant Kir4.1, the results also suggest mechanisms underlying K+ buffering in glial cells: When [K+]o is rapidly increased, as would occur during neuronal excitation, "fast block" would be relieved, promoting potassium influx to glial cells. Increase in [K+]in would then lead to relief of "slow block," further promoting K+-influx.
Figures






Similar articles
-
Inwardly rectifying K+ channel in retinal Müller cells: comparison with the KAB-2/Kir4.1 channel expressed in HEK293T cells.Jpn J Physiol. 1998 Feb;48(1):71-80. doi: 10.2170/jjphysiol.48.71. Jpn J Physiol. 1998. PMID: 9538292
-
Functional expression of Kir 6.1/SUR1-K(ATP) channels in frog retinal Müller glial cells.Glia. 2002 May;38(3):256-67. doi: 10.1002/glia.10073. Glia. 2002. PMID: 11968063
-
Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions.J Cell Mol Med. 2006 Jan-Mar;10(1):33-44. doi: 10.1111/j.1582-4934.2006.tb00289.x. J Cell Mol Med. 2006. PMID: 16563220 Free PMC article. Review.
-
Spatial distribution of spermine/spermidine content and K(+)-current rectification in frog retinal glial (Müller) cells.Glia. 2000 Jul;31(1):84-90. doi: 10.1002/(sici)1098-1136(200007)31:1<84::aid-glia80>3.0.co;2-7. Glia. 2000. PMID: 10816609
-
Potassium buffering in the central nervous system.Neuroscience. 2004;129(4):1045-56. doi: 10.1016/j.neuroscience.2004.06.008. Neuroscience. 2004. PMID: 15561419 Free PMC article. Review.
Cited by
-
New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling.J Neurosci. 2015 Oct 14;35(41):13827-35. doi: 10.1523/JNEUROSCI.2603-15.2015. J Neurosci. 2015. PMID: 26468182 Free PMC article. Review.
-
The involvement of polyamine uptake and synthesis pathways in the proliferation of neonatal astrocytes.Amino Acids. 2020 Aug;52(8):1169-1180. doi: 10.1007/s00726-020-02881-w. Epub 2020 Aug 20. Amino Acids. 2020. PMID: 32816168 Free PMC article.
-
Novel KCNJ10 Gene Variations Compromise Function of Inwardly Rectifying Potassium Channel 4.1.J Biol Chem. 2016 Apr 1;291(14):7716-26. doi: 10.1074/jbc.M115.679910. Epub 2016 Feb 11. J Biol Chem. 2016. PMID: 26867573 Free PMC article.
-
Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3.Mol Pharm. 2013 Apr 1;10(4):1450-8. doi: 10.1021/mp400024d. Epub 2013 Mar 19. Mol Pharm. 2013. PMID: 23458604 Free PMC article.
-
Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body.Biomolecules. 2022 Mar 25;12(4):501. doi: 10.3390/biom12040501. Biomolecules. 2022. PMID: 35454090 Free PMC article. Review.
References
-
- Amedee T, Roberts A, Coles JA. 1997. Potassium homeostasis and glial energy metabolism. Glia 21:46–55. - PubMed
-
- Antonov SM, Skatchkov SN, Krusek J, Veh RW, Pannicke T, Nichols CG, Reichenbach A, Eaton MJ. 2003. Two distinct sites of spermine block in Kir4.1 channels. In 33rd Annual Meeting of the Society for Neuroscience, New Orleans, LA.
-
- Biedermann B, Skatchkov SN, Bringmann A, Pannicke T, Veh R, Bernstein H-G, Reichenbach A. 1998. Spermine/Spermidine is expressed by retinal glial (Müller) cells, and controls distinct K+ channels of their membrane. Glia 23:209–220. - PubMed
-
- Bowie D, Mayer ML. 1995. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15:453–462. - PubMed
-
- Bringmann A, Pannicke T, Francke M, Wiedemann P, Skatchkov SN, Osborn NN, Reichenbach A. 2006. Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25:397–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS48201/NS/NINDS NIH HHS/United States
- S11 NS048201/NS/NINDS NIH HHS/United States
- G12 MD007583/MD/NIMHD NIH HHS/United States
- G12RR03035/RR/NCRR NIH HHS/United States
- GM50695/GM/NIGMS NIH HHS/United States
- SC2 GM095410/GM/NIGMS NIH HHS/United States
- R01 HL054171/HL/NHLBI NIH HHS/United States
- NS039408/NS/NINDS NIH HHS/United States
- S06 GM050695/GM/NIGMS NIH HHS/United States
- G12 RR003035/RR/NCRR NIH HHS/United States
- U54 NS039408/NS/NINDS NIH HHS/United States
- SC1 GM088019/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

