Why thrombin PAR1 receptors are important to the cardiac surgical patient
- PMID: 18293826
- PMCID: PMC4680704
Why thrombin PAR1 receptors are important to the cardiac surgical patient
Abstract
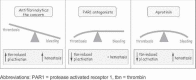
Targeting of the high-affinity thrombin receptor protease-activated receptor-1 (PAR1) on platelets represents an exciting strategy to curb the pro-thrombotic complications of cardiac surgery without interfering with the hemostatic benefits of thrombin in the coagulation cascade. The first dedicated PAR1 antagonist to complete safety trials this year has justified expectations, showing no increased risk of bleeding when added to standard anti-platelet therapy but halving major adverse cardiovascular events after percutaneous coronary intervention. In the setting of cardiothoracic surgery with cardiopulmonary bypass, an FDA-approved drug already exists with anti-PAR1 properties: aprotinin has been shown to inhibit thrombin-induced platelet activation in vitro and clinically, through sparing of PAR1 receptor cleavage and activation. Because aprotinin also exerts anti-fibrinolytic effects through blockade of plasmin, this indicates a subtle clinical mechanism of action that is simultaneously anti-thrombotic yet hemostatic. PAR1 antagonists would also be expected to exert anti-inflammatory properties through targeting of PAR1 on endothelium, and this principle has been validated in vitro for aprotinin and newer peptidomimetric antagonists. PAR1 antagonism is likely to remain an active and exciting area of research in cardiac surgery, with newer generations of PAR1 antagonists and recombinant aprotinin variants entering clinical development.
Figures
References
-
- Vu T-KH, Hung DT, Wheaton VI, Coughlin SR.. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. - PubMed
-
- Vu T-KH, Wheaton VI, Hung DT, Charo I, Coughlin SR.. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–7. - PubMed
-
- Landis RC.. Protease activated receptors: clinical relevance to hemostasis and inflammation. Hematol Oncol Clin North Am. 2007;21:103–13. - PubMed
-
- Oikonomopoulou K, Hansen KK, Saifeddine M, et al. . Proteinaseactivated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
