Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors
- PMID: 18294631
- PMCID: PMC2394572
- DOI: 10.1016/j.exer.2008.01.004
Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors
Abstract
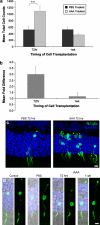
Retinal degeneration is the leading cause of untreatable blindness in the developed world. Cell transplantation strategies provide a novel therapeutic approach to repair the retina and restore sight. Previously, we have shown that photoreceptor precursor cells can integrate and form functional photoreceptors after transplantation into the subretinal space of the adult mouse. In a clinical setting, however, it is likely that far greater numbers of integrated photoreceptors would be required to restore visual function. We therefore sought to assess whether the outer limiting membrane (OLM), a natural barrier between the subretinal space and the outer nuclear layer (ONL), could be reversibly disrupted and if disruption of this barrier could lead to enhanced numbers of transplanted photoreceptors integrating into the ONL. Transient chemical disruption of the OLM was induced in adult mice using the glial toxin, dl-alpha-aminoadipic acid (AAA). Dissociated early post-natal neural retinal cells were transplanted via subretinal injection at various time-points after AAA administration. At 3 weeks post-injection, the number of integrated, differentiated photoreceptor cells was assessed and compared with those found in the PBS-treated contralateral eye. We demonstrate for the first time that the OLM can be reversibly disrupted in adult mice, using a specific dose of AAA administered by intravitreal injection. In this model, OLM disruption is maximal at 72 h, and recovers by 2 weeks. When combined with cell transplantation, disruption of the OLM leads to a significant increase in the number of photoreceptors integrated within the ONL compared with PBS-treated controls. This effect was only seen in animals in which AAA had been administered 72 h prior to transplantation, i.e. when precursor cells were delivered into the subretinal space at a time coincident with maximal OLM disruption. These findings suggest that the OLM presents a physical barrier to photoreceptor integration following transplantation into the subretinal space in the adult mouse. Reversible disruption of the OLM may provide a strategy for increasing cell integration in future therapeutic applications.
Figures




References
-
- Ahmad I., Tang L., Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000;270:517–521. - PubMed
-
- Akimoto M., Cheng H., Zhu D., Brzezinski J.A., Khanna R., Filippova E., Oh E.C., Jing Y., Linares J.L., Brooks M., Zareparsi S., Mears A.J., Hero A., Glaser T., Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl. Acad. Sci. USA. 2006;103:3890–3895. - PMC - PubMed
-
- Bignami A., Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp. Eye Res. 1979;28:63–69. - PubMed
-
- Bjorklund H., Bignami A., Dahl D. Immunohistochemical demonstration of glial fibrillary acidic protein in normal rat Muller glia and retinal astrocytes. Neurosci. Lett. 1985;54:363–368. - PubMed
-
- Chacko D.M., Rogers J.A., Turner J.E., Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem. Biophys. Res. Commun. 2000;268:842–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

