Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium
- PMID: 18295334
- PMCID: PMC2632959
- DOI: 10.1016/j.molimm.2007.12.025
Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium
Abstract
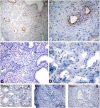
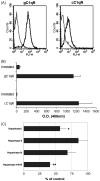
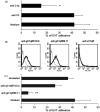
This study was prompted by the observation that decidual endothelial cells (DECs), unlike endothelial cells (ECs) of blood vessels in normal skin, kidney glomeruli and brain, express surface-bound C1q in physiologic pregnancy. This finding was unexpected, because deposits of C1q are usually observed in pathologic conditions and are associated with complement activation. In the case of DECs, we failed to detect immunoglobulins and C4 co-localized with C1q on the cell surface. Surprisingly, DECs expressed mRNA for the three chains of C1q and secreted detectable level of this component in serum-free medium. The ability to synthesize C1q is acquired by DECs during pregnancy and is not shared by ECs obtained from endometrium and from other sources. Cell-associated C1q has a molecular weight similar to that of secreted C1q and is released from DECs following treatment with heparinase or incubation at low pH. This suggests that C1q binds to DECs and it is not constitutively expressed on the cell surface. C1q is localized at contact sites between endovascular trophoblast and DECs and acts as an intercellular molecular bridge because adhesion of endovascular trophoblast to DECs was inhibited by antibodies to C1q and to a receptor recognizing its globular portion expressed on trophoblast.
Figures




References
-
- Almeda S, Rosenberg RD, Bing DH. The binding properties of human complement component C1q Interaction with mucopolysaccharides. J. Biol. Chem. 1983;258:785–791. - PubMed
-
- Bing DH, Spurlock SE, Bern MM. Synthesis of the first component of complement by primary cultures of human tumors of the colon and urogenital tract and comparable normal tissue. Clin. Immunol. Immunopathol. 1975;4:341–351. - PubMed
-
- Bossi F, Fischetti F, Pellis V, Bulla R, Ferrero E, Mollnes TE, Regoli D, Tedesco F. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J. Immunol. 2004;173:6921–6927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

