PKCdelta regulates the stimulation of vascular endothelial factor mRNA translation by angiotensin II through hnRNP K
- PMID: 18295448
- PMCID: PMC2359224
- DOI: 10.1016/j.cellsig.2008.01.016
PKCdelta regulates the stimulation of vascular endothelial factor mRNA translation by angiotensin II through hnRNP K
Abstract
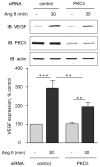
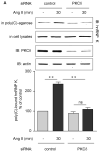
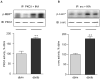
Angiotensin II (Ang II)-induced renal injury is partly mediated by growth factors such as VEGF. We have previously shown that Ang II rapidly increases VEGF protein synthesis in proximal tubular epithelial (MCT) cells by augmenting mRNA translation, which is partly dependent on activation and binding of hnRNP K to 3' untranslated region (UTR) of VEGF mRNA. Regulation of hnRNP K activation by PKCdelta was studied in MCT cells. Transfection with a PKCdelta siRNA inhibited hnRNP K Ser302 phosphorylation and activation, and reduced Ang II stimulation of VEGF synthesis. Inhibition of PKCdelta with röttlerin also prevented binding of hnRNP K to VEGF mRNA and reduced the efficiency of VEGF mRNA translation. In db/db mice at 2 weeks of type 2 diabetes, VEGF expression was increased, which was due not to increase in transcription but to augmented translation of VEGF mRNA. Augmented VEGF expression was associated with increased binding of hnRNP K to VEGF mRNA. c-src and PKCdelta activities and hnRNP K phosphorylation on Ser302 in renal cortex of db/db mice were increased compared to control mice. We conclude: Ang II-induced VEGF mRNA translation is associated with activation of hnRNP K in MCT cells. In the signaling pathway leading to hnRNP K activation induced by Ang II, PKCdelta is downstream of c-src. PKCdelta-mediated phosphorylation of hnRNP K is required for Ang II stimulation of VEGF mRNA translation. In mice with type 2 diabetes, src and PKCdelta activation and hnRNP K phosphorylation correlate with increased VEGF mRNA translation and kidney hypertrophy. 3' UTR events are important in regulation of VEGF expression in models of renal injury.
Figures












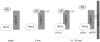
Similar articles
-
Heterogeneous nuclear ribonucleoprotein K contributes to angiotensin II stimulation of vascular endothelial growth factor mRNA translation.Am J Physiol Renal Physiol. 2007 Aug;293(2):F607-15. doi: 10.1152/ajprenal.00497.2006. Epub 2007 Jun 20. Am J Physiol Renal Physiol. 2007. PMID: 17581920
-
Acute hyperglycemia rapidly stimulates VEGF mRNA translation in the kidney. Role of angiotensin type 2 receptor (AT2).Cell Signal. 2010 Dec;22(12):1849-57. doi: 10.1016/j.cellsig.2010.07.012. Epub 2010 Jul 24. Cell Signal. 2010. PMID: 20667471 Free PMC article.
-
Vascular endothelial growth factor induces protein synthesis in renal epithelial cells: a potential role in diabetic nephropathy.Kidney Int. 2003 Aug;64(2):468-79. doi: 10.1046/j.1523-1755.2003.00135.x. Kidney Int. 2003. PMID: 12846742
-
Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species.Am J Physiol Renal Physiol. 2006 Apr;290(4):F927-36. doi: 10.1152/ajprenal.00331.2005. Epub 2005 Oct 25. Am J Physiol Renal Physiol. 2006. PMID: 16249273
-
Translational regulation of vascular endothelial growth factor expression in renal epithelial cells by angiotensin II.Am J Physiol Renal Physiol. 2005 Mar;288(3):F521-9. doi: 10.1152/ajprenal.00271.2004. Epub 2004 Nov 30. Am J Physiol Renal Physiol. 2005. PMID: 15572520
Cited by
-
Heterogeneous nuclear ribonucleoprotein K, an RNA-binding protein, is required for optic axon regeneration in Xenopus laevis.J Neurosci. 2012 Mar 7;32(10):3563-74. doi: 10.1523/JNEUROSCI.5197-11.2012. J Neurosci. 2012. PMID: 22399778 Free PMC article.
-
Mechanism of VEGF expression by high glucose in proximal tubule epithelial cells.Mol Cell Endocrinol. 2010 Jan 15;314(1):136-42. doi: 10.1016/j.mce.2009.09.009. Epub 2009 Sep 16. Mol Cell Endocrinol. 2010. PMID: 19765632 Free PMC article.
-
Post-translational modification control of RNA-binding protein hnRNPK function.Open Biol. 2019 Mar 29;9(3):180239. doi: 10.1098/rsob.180239. Open Biol. 2019. PMID: 30836866 Free PMC article. Review.
-
Glycoxidised LDL induced the upregulation of Axl receptor tyrosine kinase and its ligand in mouse mesangial cells.PLoS One. 2012;7(11):e50297. doi: 10.1371/journal.pone.0050297. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226259 Free PMC article.
-
Regulation of mRNA translation in renal physiology and disease.Am J Physiol Renal Physiol. 2009 Nov;297(5):F1153-65. doi: 10.1152/ajprenal.90748.2008. Epub 2009 Jun 17. Am J Physiol Renal Physiol. 2009. PMID: 19535566 Free PMC article. Review.
References
-
- Rincon-Choles H, Kasinath BS, Gorin Y, Abboud HE. Kidney Int Suppl. 2002:8–11. - PubMed
-
- Feliers D, Duraisamy S, Barnes JL, Ghosh-Choudhury G, Kasinath BS. Am J Physiol Renal Physiol. 2005;288:F521–529. - PubMed
-
- Feliers D, Lee MJ, Ghosh-Choudhury G, Bomsztyk K, Kasinath BS. Am J Physiol Renal Physiol. 2007;293:F607–615. - PubMed
-
- Ostrowski J, Schullery DS, Denisenko ON, Higaki Y, Watts J, Aebersold R, Stempka L, Gschwendt M, Bomsztyk K. J Biol Chem. 2000;275:3619–3628. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

