A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis
- PMID: 18295755
- PMCID: PMC2373417
- DOI: 10.1016/j.ydbio.2008.01.012
A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis
Abstract
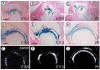
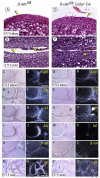
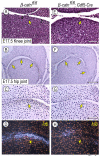
The origin, roles and fate of progenitor cells forming synovial joints during limb skeletogenesis remain largely unclear. Here we produced prenatal and postnatal genetic cell fate-maps by mating ROSA-LacZ-reporter mice with mice expressing Cre-recombinase at prospective joint sites under the control of Gdf5 regulatory sequences (Gdf5-Cre). Reporter-expressing cells initially constituted the interzone, a compact mesenchymal structure representing the first overt sign of joint formation, and displayed a gradient-like distribution along the ventral-to-dorsal axis. The cells expressed genes such as Wnt9a, Erg and collagen IIA, remained predominant in the joint-forming sites over time, gave rise to articular cartilage, synovial lining and other joint tissues, but contributed little if any to underlying growth plate cartilage and shaft. To study their developmental properties more directly, we isolated the joint-forming cells from prospective autopod joint sites using a novel microsurgical procedure and tested them in vitro. The cells displayed a propensity to undergo chondrogenesis that was enhanced by treatment with exogenous rGdf5 but blocked by Wnt9a over-expression. To test roles for such Wnt-mediated anti-chondrogenic capacity in vivo, we created conditional mutants deficient in Wnt/beta-catenin signaling using Col2-Cre or Gdf5-Cre. Synovial joints did form in both mutants; however, the joints displayed a defective flat cell layer normally abutting the synovial cavity and expressed markedly reduced levels of lubricin. In sum, our data indicate that cells present at prospective joint sites and expressing Gdf5 constitute a distinct cohort of progenitor cells responsible for limb joint formation. The cells appear to be patterned along specific limb symmetry axes and rely on local signaling tools to make distinct contributions to joint formation.
Figures






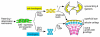
References
-
- Barna M, Pandolfi PP, Niswander L. Gli3 and Plzf cooperate in proximal limb patterning at early stages of limb development. Nature. 2005;436:277–281. - PubMed
-
- Bird HA. Controversies in the treatment of osteoarthritis. Clin. Rheum. 2003;22:165–167. - PubMed
-
- Bland YA, Ashhurst d. E. Development and ageing of the articular cartilage of the rabbit knee joint: distribution of fibrillar collagens. Anat. Embryol. 1996;194:607–619. - PubMed
-
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

