Autoantibodies from mice exposed to Libby amphibole asbestos bind SSA/Ro52-enriched apoptotic blebs of murine macrophages
- PMID: 18295955
- PMCID: PMC2346587
- DOI: 10.1016/j.tox.2008.01.008
Autoantibodies from mice exposed to Libby amphibole asbestos bind SSA/Ro52-enriched apoptotic blebs of murine macrophages
Abstract
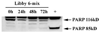
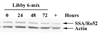
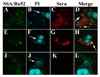
Asbestos exposure is associated with increased autoimmune responses in humans. For example, in Libby, MT where significant asbestos exposure has occurred due to an asbestos-contaminated vermiculite mine near the community, residents have developed increased autoimmune responses compared to an unexposed population. However, the exact mechanism by which Libby amphibole asbestos generates autoimmune responses is unclear. A murine model of amphibole asbestos-induced autoimmunity was recently established, and one of the targets of the autoantibodies (AAs) was the SSA/Ro52 autoantigen. The purpose of this study was to determine whether the SSA/Ro52 autoantigen is exposed at the surface of cells as a result of asbestos exposure as a possible mechanism leading to antigenicity. Our results indicate that Libby asbestos induces apoptosis in murine macrophages as determined by phosphatidylserine exposure, cleavage of poly(ADP-ribose) polymerase and morphological changes such as nuclear condensation. Moreover, asbestos-induced apoptosis results in the formation of apoptotic cell surface blebs enriched in SSA/Ro52 as determined by confocal microscopy. Most importantly, apoptotic cell surface blebs are recognized by AAs from mice exposed to amphibole asbestos suggesting that these cell surface structures may be antigenic when presented in a pro-inflammatory context. This study supports the hypothesis that the induction of apoptosis plays a key role in environmentally induced autoimmunity through cell surface exposure of a known autoantigen.
Figures




Similar articles
-
Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages.Toxicol Sci. 2007 Sep;99(1):277-88. doi: 10.1093/toxsci/kfm166. Epub 2007 Jun 19. Toxicol Sci. 2007. PMID: 17578862
-
Erionite induces production of autoantibodies and IL-17 in C57BL/6 mice.Toxicol Appl Pharmacol. 2014 Mar 15;275(3):257-64. doi: 10.1016/j.taap.2014.01.018. Epub 2014 Feb 9. Toxicol Appl Pharmacol. 2014. PMID: 24518925 Free PMC article.
-
Comparative health effects in mice of Libby amphibole asbestos and a fibrous amphibole from Arizona.Toxicol Appl Pharmacol. 2017 Nov 1;334:24-34. doi: 10.1016/j.taap.2017.08.022. Epub 2017 Sep 8. Toxicol Appl Pharmacol. 2017. PMID: 28870655
-
Amphibole asbestos in tree bark--a review of findings for this inhalational exposure source in Libby, Montana.J Occup Environ Hyg. 2012;9(6):387-97. doi: 10.1080/15459624.2012.682217. J Occup Environ Hyg. 2012. PMID: 22577793 Review.
-
A review of scientific literature examining the mining history, geology, mineralogy, and amphibole asbestos health effects of the Rainy Creek igneous complex, Libby, Montana, USA.Inhal Toxicol. 2006 Nov;18(12):949-62. doi: 10.1080/08958370600834982. Inhal Toxicol. 2006. PMID: 16920668 Review.
Cited by
-
Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus.Lupus. 2014 May;23(6):527-36. doi: 10.1177/0961203313511680. Lupus. 2014. PMID: 24763537 Free PMC article. Review.
-
Advances in biological functions and applications of apoptotic vesicles.Cell Commun Signal. 2023 Sep 25;21(1):260. doi: 10.1186/s12964-023-01251-9. Cell Commun Signal. 2023. PMID: 37749626 Free PMC article. Review.
-
Exposure to silicates and systemic autoimmune-related outcomes in rodents: a systematic review.Part Fibre Toxicol. 2022 Jan 7;19(1):4. doi: 10.1186/s12989-021-00439-6. Part Fibre Toxicol. 2022. PMID: 34996462 Free PMC article.
-
Autoimmunity and asbestos exposure.Autoimmune Dis. 2014;2014:782045. doi: 10.1155/2014/782045. Epub 2014 Apr 29. Autoimmune Dis. 2014. PMID: 24876951 Free PMC article. Review.
-
Meeting report: mode(s) of action of asbestos and related mineral fibers.Environ Health Perspect. 2011 Dec;119(12):1806-10. doi: 10.1289/ehp.1003240. Epub 2011 Aug 1. Environ Health Perspect. 2011. PMID: 21807578 Free PMC article.
References
-
- Blake DJ, Bolin CM, Cox DP, Cardozo-Pelaez F, Pfau JC. Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxicological Sciences. 2007;99:277–288. - PubMed
-
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. - PubMed
-
- Bondanza A, Zimmermann VS, Dell'Antonio G, Cin ED, Balestrieri G, Tincani A, Amoura Z, Piette JC, Sabbadini MG, Rovere-Querini P, Manfredi AA. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum. 2004;50:1549–1560. - PubMed
-
- Brown JM, Pfau JC, Pershouse MA, Holian A. Silica, Apoptosis and Autoimmunity. Journal of Immunotoxicology. 2004;1:177–187. - PubMed
-
- Brown JM, Schwanke CM, Pershouse MA, Pfau JC, Holian A. Effects of rottlerin on silica-exacerbated systemic autoimmune disease in New Zealand mixed mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L990–L998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

