HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice
- PMID: 18296443
- PMCID: PMC2431057
- DOI: 10.1074/jbc.M707572200
HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice
Abstract
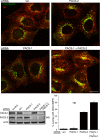
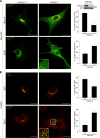
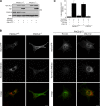
Human immunodeficiency virus, type 1, negative factor (Nef) initiates down-regulation of cell-surface major histocompatibility complex-I (MHC-I) by assembling an Src family kinase (SFK)-ZAP70/Syk-phosphoinositide 3-kinase (PI3K) cascade through the sequential actions of two sites, Nef EEEE(65) and PXXP(75). The internalized MHC-I molecules are then sequestered in endosomal compartments by a process requiring Nef Met(20). How Nef assembles the multikinase cascade to trigger the MHC-I down-regulation pathway is unknown. Here we report that EEEE(65)-dependent binding to the sorting protein PACS-2 targets Nef to the paranuclear region, enabling PXXP(75) to bind and activate a trans-Golgi network (TGN)-localized SFK. This SFK then phosphorylates ZAP-70 to recruit class I PI3K by interaction with the p85 C-terminal Src homology 2 domain. Using splenocytes and embryonic fibroblasts from PACS-2(-/-) mice, we confirm genetically that Nef requires PACS-2 to localize to the paranuclear region and assemble the multikinase cascade. Moreover, genetic loss of PACS-2 or inhibition of class I PI3K prevents Nef-mediated MHC-I down-regulation, demonstrating that short interfering RNA knockdown of PACS-2 phenocopies the gene knock-out. This PACS-2-dependent targeting pathway is not restricted to Nef, because PACS-2 is also required for trafficking of an endocytosed cation-independent mannose 6-phosphate receptor reporter from early endosomes to the TGN. Together, these results demonstrate PACS-2 is required for Nef action and sorting of itinerant membrane cargo in the TGN/endosomal system.
Figures





References
-
- Das, S. R., and Jameel, S. (2005) Indian J. Med. Res. 121 315-332 - PubMed
-
- Peterlin, B. M., and Trono, D. (2003) Nat. Rev. Immunol. 3 97-107 - PubMed
-
- Stevenson, M. (2003) Nat. Med. 9 853-860 - PubMed
-
- Stove, V., Naessens, E., Stove, C., Swigut, T., Plum, J., and Verhasselt, B. (2003) Blood 102 2925-2932 - PubMed
-
- Thoulouze, M. I., Sol-Foulon, N., Blanchet, F., Dautry-Varsat, A., Schwartz, O., and Alcover, A. (2006) Immunity 24 547-561 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

