Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism
- PMID: 18296633
- PMCID: PMC2265166
- DOI: 10.1073/pnas.0800165105
Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism
Abstract
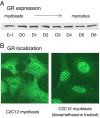
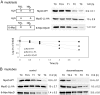
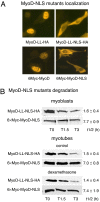
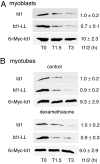
Accelerated protein degradation via the ubiquitin-proteasome pathway is the principal cause of skeletal muscle wasting associated with common human disease states and pharmacological treatment with glucocorticoids. Although many protein regulatory factors essential for muscle development and regeneration are degraded via the ubiquitin system, little is known about the mechanisms and regulation of this pathway that promote wasting muscle. Here, we demonstrate that, in differentiated myotubes, glucocorticoid, via the glucocorticoid receptor, selectively induces a decrease in protein abundance of MyoD, a master switch for muscle development and regeneration, but not that of its negative regulator Id1. This decrease in MyoD protein results from accelerated degradation after glucocorticoid exposure. Using MyoD and Id1 mutants deficient in either N terminus-dependent or internal lysine-dependent ubiquitination, we further show that these ubiquitination pathways of MyoD degradation are regulated differently from those of Id1 degradation. Specifically, glucocorticoid activates the N-terminal ubiquitination pathway in MyoD degradation in myotubes, without concomitant effects on Id1 degradation. This effect of glucocorticoid on MyoD and Id1 protein degradation is associated with the distinct cellular compartments in which their degradation occurs. Taken together, these results support a key role for the N terminus-dependent ubiquitination pathway in the physiology of muscle protein degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hong DH, Forsberg NE. Effects of dexamethasone on protein degradation and protease gene expression in rat L8 myotube cultures. Mol Cell Endocrinol. 1995;108:199–209. - PubMed
-
- Thompson MG, et al. Stimulation of myofibrillar protein degradation and expression of mRNA encoding the ubiquitin–proteasome system in C (2)C(12) myotubes by dexamethasone: Effect of the proteasome inhibitor MG-132. J Cell Physiol. 1999;181:455–461. - PubMed
-
- Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock. 1998;10:298–306. - PubMed
-
- Bodine SC, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

