Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance
- PMID: 18296638
- PMCID: PMC2265194
- DOI: 10.1073/pnas.0712275105
Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance
Abstract
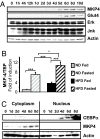
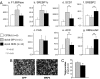
Insulin resistance, a hallmark of type 2 diabetes and obesity, is associated with increased activity of MAP and stress-activated protein (SAP) kinases, which results in decreased insulin signaling. Our goal was to investigate the role of MAP kinase phosphatase-4 (MKP-4) in modulating this process. We found that MKP-4 expression is up-regulated during adipocyte and myocyte differentiation in vitro and up-regulated during fasting in white adipose tissue in vivo. Overexpression of MKP-4 in 3T3-L1 cells inhibited ERK and JNK phosphorylation and, to a lesser extent, p38MAPK phosphorylation. As a result, the phosphorylation of IRS-1 serine 307 induced by anisomycin was abolished, leading to a sensitization of insulin signaling with recovery of insulin-stimulated IRS-1 tyrosine phosphorylation, IRS-1 docking with phosphatidylinositol 3-kinase, and Akt phosphorylation. MKP-4 also reversed the effect of TNF-alpha to inhibit insulin signaling; alter IL-6, Glut1 and Glut4 expression; and inhibit insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Overexpression of MKP-4 in the liver of ob/ob mice decreased ERK and JNK phosphorylation, leading to a reduction in fed and fasted glycemia, improved glucose intolerance, decreased expression of gluconeogenic and lipogenic genes, and reduced hepatic steatosis. Thus, MKP-4 has a protective effect against the development of insulin resistance through its ability to dephosphorylate and inactivate crucial mediators of stress-induced insulin resistance, such as ERK and JNK, and increasing MKP-4 activity might provide a therapy for insulin-resistant disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


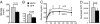
References
-
- Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE. Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3–L1 adipocytes. Mol Endocrinol. 2000;14:1557–1569. - PubMed
-
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–9054. - PubMed
-
- Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. - PubMed
-
- Fujishiro M, et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3–L1 adipocytes. Mol Endocrinol. 2003;17:487–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

