PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains
- PMID: 18296639
- PMCID: PMC2265113
- DOI: 10.1073/pnas.0712235105
PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains
Abstract
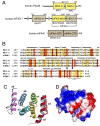
Programmed Cell Death 4 (PDCD4) is a protein known to bind eukaryotic initiation factor 4A (eIF4A), inhibit translation initiation, and act as a tumor suppressor. PDCD4 contains two C-terminal MA3 domains, which are thought to be responsible for its inhibitory function. Here, we analyze the structures and inhibitory functions of these two PDCD4 MA3 domains by x-ray crystallography, NMR, and surface plasmon resonance. We show that both MA3 domains are structurally and functionally very similar and bind specifically to the eIF4A N-terminal domain (eIF4A-NTD) using similar binding interfaces. We found that the PDCD4 MA3 domains compete with the eIF4G MA3 domain and RNA for eIF4A binding. Our data provide evidence that PDCD4 inhibits translation initiation by displacing eIF4G and RNA from eIF4A. The PDCD4 MA3 domains act synergistically to form a tighter and more stable complex with eIF4A, which explains the need for two tandem MA3 domains.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

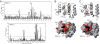


References
-
- Shibahara K, et al. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166:297–301. - PubMed
-
- Jansen AP, Camalier CE, Stark C, Colburn NH. Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther. 2004;3:103–110. - PubMed
-
- Dorrello NV, et al. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. - PubMed
-
- Goke A, et al. DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem Biophys Res Commun. 2002;297:78–82. - PubMed
-
- Kang MJ, et al. Up-regulation of PDCD4 in senescent human diploid fibroblasts. Biochem Biophys Res Commun. 2002;293:617–621. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

