Hypochlorite-modified high-density lipoprotein acts as a sink for myeloperoxidase in vitro
- PMID: 18296711
- PMCID: PMC4060512
- DOI: 10.1093/cvr/cvn051
Hypochlorite-modified high-density lipoprotein acts as a sink for myeloperoxidase in vitro
Abstract
Aims: Myeloperoxidase (MPO), a cardiovascular risk factor in humans, is an in vivo catalyst for lipoprotein modification via intermediate formation of reactive chlorinating species. Among the different lipoprotein classes, anti-atherogenic high-density lipoprotein (HDL) represents a major target for modification by hypochlorous acid (HOCl), generated from H2O2 by MPO in the presence of physiological chloride concentrations. As MPO was identified as an HDL-associated protein that could facilitate selective oxidative modification of its physiological carrier, the aim of the present study was to investigate whether and to what extent modification of HDL by HOCl affects the binding affinity of MPO in vitro.
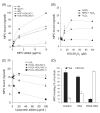
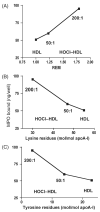
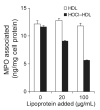
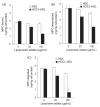
Methods and results: We show that binding affinity of 125I-labelled MPO to HDL markedly increases as a function of increasing extent of HOCl modification of HDL. In contrast to native HDL, HOCl-HDL potently inhibits MPO binding/uptake by endothelial cells and effectively attenuates metabolism of MPO by macrophages. Reduction of HDL-associated chloramines with methionine strongly impaired binding affinity of MPO towards HOCl-HDL. This indicates that N-chloramines generated by HOCl are regulators of the high-affinity interaction between HOCl-HDL and positively charged MPO. Most importantly, the presence of HOCl-HDL is almost without effect on the halogenating activity of MPO.
Conclusion: We propose that MPO-dependent modification of HDL and concomitant increase in the binding affinity for MPO could generate a vicious cycle of MPO transport to and MPO-dependent modification at sites of chronic inflammation.
Figures


Similar articles
-
Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport.J Biol Chem. 2002 Aug 30;277(35):32172-9. doi: 10.1074/jbc.M200503200. Epub 2002 Jun 17. J Biol Chem. 2002. PMID: 12070141
-
2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis.Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2302-6. doi: 10.1161/01.ATV.0000148703.43429.25. Epub 2004 Oct 28. Arterioscler Thromb Vasc Biol. 2004. PMID: 15514213
-
Plasma phospholipid transfer protein-mediated reactions are impaired by hypochlorite-modification of high density lipoprotein.Int J Biochem Cell Biol. 2003 Feb;35(2):192-202. doi: 10.1016/s1357-2725(02)00130-9. Int J Biochem Cell Biol. 2003. PMID: 12479869
-
Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins.Arch Biochem Biophys. 2006 Jan 15;445(2):245-55. doi: 10.1016/j.abb.2005.08.008. Epub 2005 Aug 31. Arch Biochem Biophys. 2006. PMID: 16171772 Review.
-
Myeloperoxidase in kidney disease.Kidney Int. 2003 Dec;64(6):1956-67. doi: 10.1046/j.1523-1755.2003.00336.x. Kidney Int. 2003. PMID: 14633118 Review.
Cited by
-
VPO1 mediates oxidation of LDL and formation of foam cells.Oncotarget. 2016 Jun 14;7(24):35500-35511. doi: 10.18632/oncotarget.9193. Oncotarget. 2016. PMID: 27167346 Free PMC article.
-
Oxidized high-density lipoprotein impairs endothelial progenitor cells' function by activation of CD36-MAPK-TSP-1 pathways.Antioxid Redox Signal. 2015 Feb 1;22(4):308-24. doi: 10.1089/ars.2013.5743. Epub 2014 Dec 2. Antioxid Redox Signal. 2015. PMID: 25313537 Free PMC article.
-
Protein carbamylation renders high-density lipoprotein dysfunctional.Antioxid Redox Signal. 2011 Jun 15;14(12):2337-46. doi: 10.1089/ars.2010.3640. Epub 2011 Mar 28. Antioxid Redox Signal. 2011. PMID: 21235354 Free PMC article.
-
Reactive oxygen species in cardiovascular disease.Free Radic Biol Med. 2011 Sep 1;51(5):978-92. doi: 10.1016/j.freeradbiomed.2011.05.004. Epub 2011 May 15. Free Radic Biol Med. 2011. PMID: 21627987 Free PMC article. Review.
-
Understanding Myeloperoxidase-Induced Damage to HDL Structure and Function in the Vessel Wall: Implications for HDL-Based Therapies.Antioxidants (Basel). 2022 Mar 15;11(3):556. doi: 10.3390/antiox11030556. Antioxidants (Basel). 2022. PMID: 35326206 Free PMC article. Review.
References
-
- Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. - PubMed
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. - PubMed
-
- Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase–hydrogen peroxide–chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. - PubMed
-
- van den Berg JJ, Winterbourn CC, Kuypers FA. Hypochlorous acid-mediated modification of cholesterol and phospholipid: analysis of reaction products by gas chromatography–mass spectrometry. J Lipid Res. 1993;34:2005–2012. - PubMed
-
- Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332:110–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

