Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast
- PMID: 18296751
- PMCID: PMC2311445
- DOI: 10.1194/jlr.R800003-JLR200
Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast
Abstract
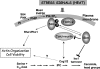
Our understanding of sphingolipid metabolism and functions in the baker's yeast Saccharomyces cerevisiae has progressed substantially in the past 2 years. Yeast sphingolipids contain a C26-acyl moiety, all of the genes necessary to make these long-chain fatty acids have been identified, and a mechanism for how chain length is determined has been proposed. Advances in understanding how the de novo synthesis of ceramide and complex sphingolipids is regulated have been made, and they demonstrate that the Target Of Rapamycin Complex 2 (TORC2) controls ceramide synthase activity. Other work shows that TORC2 regulates the level of complex sphingolipids in a pathway using the Slm1 and Slm2 proteins to control the protein phosphatase calcineurin, which regulates the breakdown of complex sphingolipids. The activity of Slm1 and Slm2 has also been shown to be regulated during heat stress by phosphoinositides and TORC2, along with sphingoid long-chain bases and the Pkh1 and Pkh2 protein kinases, to control the actin cytoskeleton, the trafficking of nutrient transporters, and cell viability. Together, these results provide the first molecular insights into understanding previous genetic interaction data that indicated a connection between sphingolipids and the TORC2 and phosphoinositide signaling networks. This new knowledge provides a foundation for greatly advancing our understanding of sphingolipid biology in yeast.
Figures



References
-
- Hannun Y. A., and R. M. Bell. 1989. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 243 500–507. - PubMed
-
- Ogretmen B., and Y. A. Hannun. 2004. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 4 604–616. - PubMed
-
- Savtchouk I. A., F. J. Mattie, and A. A. Ollis. 2007. Ceramide: from embryos to tumors. Sci. STKE. 2007 jc1–jc2. - PubMed
-
- El Alwani M., B. X. Wu, L. M. Obeid, and Y. A. Hannun. 2006. Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol. Ther. 112 171–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

